The role of urokinase plasminogen activator and plasmin activator inhibitor-1 on vein wall remodeling in experimental deep vein thrombosis
- PMID: 22796119
- PMCID: PMC3463752
- DOI: 10.1016/j.jvs.2012.02.054
The role of urokinase plasminogen activator and plasmin activator inhibitor-1 on vein wall remodeling in experimental deep vein thrombosis
Abstract
Objective: Deep vein thrombosis (DVT) resolution instigates an inflammatory response, resulting in vessel wall damage and scarring. Urokinase-plasminogen activator (uPA) and its inhibitor, plasminogen activator inhibitor-1 (PAI-1), are integral components of the fibrinolytic system, essential for venous thrombosis (VT) resolution. This study determined the vein wall response when exposed to increased and decreased plasmin activity.
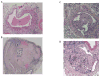
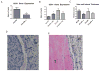
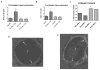
Methods: A mouse inferior vena cava (IVC) ligation model in uPA -/- or PAI-1 -/- and their genetic wild types (B6/SvEv and C57/BL6, respectively) was used to create stasis thrombi, with tissue harvest at either 8 or 21 days. Tissue analysis included gene expression of vascular smooth muscle cells (alpha smooth muscle actin [αSMA], SM22) and endothelial marker (CD31), by real-time polymerase chain reaction, enzyme-linked immunosorbent assay, matrix metalloproteinase (MMP)-2 and -9 activity by zymography, and vein wall collagen by picro-Sirius red histologic analysis. A P < .05 was considered significant.
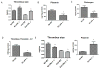
Results: Thrombi were significantly larger in both 8-day and 21-day uPA -/- as compared with wild type (WT) and were significantly smaller in both 8-day and 21-day PAI-1 -/- as compared with WT. Correspondingly, 8-day plasmin levels were reduced in half in uPA -/- and increased three-fold in PAI-1 -/- when compared with respective WT thrombi (P < .05; n = 5-6). The endothelial marker CD31 was elevated two-fold in PAI-1 -/- mice at 8 days, but reduced 2.5-fold at 21 days in uPA -/- as compared with WT (P = .02; n = 5-6), suggesting less endothelial preservation. Vein wall vascular smooth muscle cell (VSMC) gene expression showed that 8-day and 21-day PAI-1 -/- mice had 2.3- and 3.8-fold more SM22 and 1.8- and 2.3-fold more αSMA expression than respective WT (P < .05; n = 5-7), as well as 1.8-fold increased αSMA (+) cells (P ≤ .05; n = 3-5). No significant difference in MMP-2 or -9 activity was found in the PAI-1 -/- mice compared with WT, while 5.4-fold more MMP-9 was present in 21-day WT than 21-day uPA -/- (P = .03; n = 5). Lastly, collagen was ∼two-fold greater at 8 days in PAI-1 -/- IVC as compared with WT (P = .03; n = 6) with no differences observed in uPA -/- mice.
Conclusions: In stasis DVT, plasmin activity is critical for thrombus resolution. Divergent vein wall responses occur with gain or loss of plasmin activity, and despite smaller VT, greater vein wall fibrosis was associated with lack of PAI-1.
Copyright © 2012 Society for Vascular Surgery. Published by Mosby, Inc. All rights reserved.
Figures

Similar articles
-
Plasmin inhibition increases MMP-9 activity and decreases vein wall stiffness during venous thrombosis resolution.J Surg Res. 2007 Oct;142(2):357-63. doi: 10.1016/j.jss.2007.03.064. Epub 2007 Jun 14. J Surg Res. 2007. PMID: 17574586 Free PMC article.
-
Vein wall remodeling after deep vein thrombosis involves matrix metalloproteinases and late fibrosis in a mouse model.J Vasc Surg. 2005 Jul;42(1):140-8. doi: 10.1016/j.jvs.2005.04.014. J Vasc Surg. 2005. PMID: 16012463
-
Urokinase plasminogen activator independent early experimental thrombus resolution: MMP2 as an alternative mechanism.Thromb Haemost. 2010 Dec;104(6):1174-83. doi: 10.1160/TH10-03-0184. Epub 2010 Sep 30. Thromb Haemost. 2010. PMID: 20886179
-
PAI-1 in tissue fibrosis.J Cell Physiol. 2012 Feb;227(2):493-507. doi: 10.1002/jcp.22783. J Cell Physiol. 2012. PMID: 21465481 Free PMC article. Review.
-
Plasminogen activator inhibitor-1 is an aggregate response factor with pleiotropic effects on cell signaling in vascular disease and the tumor microenvironment.Thromb Res. 2010 May;125(5):377-81. doi: 10.1016/j.thromres.2009.11.034. Epub 2010 Jan 15. Thromb Res. 2010. PMID: 20079523 Free PMC article. Review.
Cited by
-
Low-molecular-weight heparin modulates vein wall fibrotic response in a plasminogen activator inhibitor 1-dependent manner.J Vasc Surg Venous Lymphat Disord. 2014 Oct 1;2(4):441-450.e1. doi: 10.1016/j.jvsv.2014.02.004. J Vasc Surg Venous Lymphat Disord. 2014. PMID: 25419511 Free PMC article.
-
Oncostatin M, Serpins, and Oxidative Stress in Extracellular Matrix Remodeling and Arteriovenous Fistula Maturation.Cardiol Cardiovasc Med. 2023;7(2):129-140. doi: 10.26502/fccm.92920318. Epub 2023 Apr 20. Cardiol Cardiovasc Med. 2023. PMID: 37484520 Free PMC article.
-
Targeting Fibrinolytic Inhibition for Venous Thromboembolism Treatment: Overview of an Emerging Therapeutic Approach.Circulation. 2024 Sep 10;150(11):884-898. doi: 10.1161/CIRCULATIONAHA.124.069728. Epub 2024 Sep 9. Circulation. 2024. PMID: 39250537 Review.
-
The effect of matrix metalloproteinase 2 and matrix metalloproteinase 2/9 deletion in experimental post-thrombotic vein wall remodeling.J Vasc Surg. 2013 Nov;58(5):1375-1384.e2. doi: 10.1016/j.jvs.2012.11.088. Epub 2013 Mar 13. J Vasc Surg. 2013. PMID: 23490298 Free PMC article.
-
Venous stasis-induced fibrinolysis prevents thrombosis in mice: role of α2-antiplasmin.Blood. 2019 Sep 19;134(12):970-978. doi: 10.1182/blood.2019000049. Epub 2019 Aug 8. Blood. 2019. PMID: 31395599 Free PMC article.
References
-
- Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, Greer IA, Heit JA, Hutchinson JL, Kakkar AK, Mottier D, Oger E, Samama MM, Spannagl M. Venous thromboembolism (vte) in europe. The number of vte events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–764. - PubMed
-
- Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. - PubMed
-
- Kahn SR, Shrier I, Julian JA, Ducruet T, Arsenault L, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Desjardins L, Lamping DL, Johri M, Ginsberg JS. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698–707. - PubMed
-
- Kahn SR, Shbaklo H, Lamping DL, Holcroft CA, Shrier I, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Desjardins L, Johri M, Ginsberg JS. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6:1105–1112. - PubMed
-
- Meissner MH, Caps MT, Zierler BK, Polissar N, Bergelin RO, Manzo RA, Strandness DE., Jr Determinants of chronic venous disease after acute deep venous thrombosis. J Vasc Surg. 1998;28:826–833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

