The role of scaffold microarchitecture in engineering endothelial cell immunomodulation
- PMID: 22796162
- PMCID: PMC3615558
- DOI: 10.1016/j.biomaterials.2012.06.052
The role of scaffold microarchitecture in engineering endothelial cell immunomodulation
Abstract
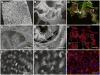
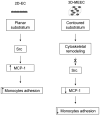
The implantation of matrix-embedded endothelial cells (MEECs) has been reported to have great therapeutic potential in controlling the vascular response to injury and maintaining patency in arteriovenous anastomoses. While there is an appreciation of their effectiveness in clinical and animal studies, the mechanisms through which they mediate these powerful effects remain relatively unknown. In this work, we examined the hypothesis that the 3-dimensional microarchitecture of the tissue engineering scaffold was a key regulator of endothelial behavior in MEEC constructs. Notably, we found that ECs in porous collagen scaffold had a markedly altered cytoskeletal structure with oriented actin fibers and rearrangement of the focal adhesion proteins in comparison to cells grown on 2D surfaces. We examined the immunomodulatory capabilities of MEECs and discovered that they were able to reduce the recruitment of monocytes to an inflamed endothelial monolayer by 5-fold compared to EC on 2D surfaces. An analysis of secreted factors from the cells revealed an 8-fold lower release of Monocyte Chemotactic Protein-1 (MCP-1) from MEECs. Differences between 3D and 2D cultured cells were abolished in the presence of inhibitors to the focal adhesion associated signaling molecule Src suggesting that adhesion-mediated signaling is essential in controlling the potent immunomodulatory effects of MEEC.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures





Similar articles
-
Tuning of collagen scaffold properties modulates embedded endothelial cell regulatory phenotype in repair of vascular injuries in vivo.Adv Healthc Mater. 2015 Oct 28;4(15):2220-8. doi: 10.1002/adhm.201500457. Epub 2015 Sep 1. Adv Healthc Mater. 2015. PMID: 26333178 Free PMC article.
-
Matrix-embedded endothelial cells are protected from the uremic milieu.Nephrol Dial Transplant. 2011 Dec;26(12):3858-65. doi: 10.1093/ndt/gfr337. Epub 2011 Jul 27. Nephrol Dial Transplant. 2011. PMID: 21795755 Free PMC article.
-
Fibrin- and collagen-based matrices attenuate inflammatory and procoagulant responses in human endothelial cell cultures exposed to Staphylococcus aureus.Tissue Eng Part A. 2012 Jan;18(1-2):147-56. doi: 10.1089/ten.TEA.2011.0029. Epub 2011 Oct 3. Tissue Eng Part A. 2012. PMID: 21806488
-
Matrix stiffness exerts biphasic control over monocyte-endothelial adhesion via Rho-mediated ICAM-1 clustering.Integr Biol (Camb). 2016 Aug 8;8(8):869-78. doi: 10.1039/c6ib00084c. Epub 2016 Jul 22. Integr Biol (Camb). 2016. PMID: 27444067
-
Immunomodulation by endothelial cells - partnering up with the immune system?Nat Rev Immunol. 2022 Sep;22(9):576-588. doi: 10.1038/s41577-022-00694-4. Epub 2022 Mar 14. Nat Rev Immunol. 2022. PMID: 35288707 Free PMC article. Review.
Cited by
-
Synergistic effects of matrix nanotopography and stiffness on vascular smooth muscle cell function.Tissue Eng Part A. 2014 Aug;20(15-16):2115-26. doi: 10.1089/ten.tea.2013.0455. Epub 2014 Apr 2. Tissue Eng Part A. 2014. PMID: 24694244 Free PMC article.
-
Unraveling and Harnessing the Immune Response at the Cell-Biomaterial Interface for Tissue Engineering Purposes.Adv Healthc Mater. 2024 Jul;13(17):e2301939. doi: 10.1002/adhm.202301939. Epub 2024 Apr 5. Adv Healthc Mater. 2024. PMID: 38217464 Free PMC article. Review.
-
In Vitro and In Vivo Characterization of N-Acetyl-L-Cysteine Loaded Beta-Tricalcium Phosphate Scaffolds.Int J Biomater. 2018 Jul 31;2018:9457910. doi: 10.1155/2018/9457910. eCollection 2018. Int J Biomater. 2018. PMID: 30151010 Free PMC article.
-
Evaluate the growth and adhesion of osteoblast cells on nanocomposite scaffold of hydroxyapatite/titania coated with poly hydroxybutyrate.Adv Biomed Res. 2016 Sep 29;5:156. doi: 10.4103/2277-9175.188486. eCollection 2016. Adv Biomed Res. 2016. PMID: 27761431 Free PMC article.
-
Increased Paracrine Immunomodulatory Potential of Mesenchymal Stromal Cells in Three-Dimensional Culture.Tissue Eng Part B Rev. 2016 Aug;22(4):322-9. doi: 10.1089/ten.TEB.2015.0532. Epub 2016 Mar 16. Tissue Eng Part B Rev. 2016. PMID: 26861485 Free PMC article. Review.
References
-
- Ribatti D, Nico B, Vacca A, Roncali L, Dammacco F. Endothelial cell heterogeneity and organ specificity. J Hematother Stem Cell Res. 2002;11(1):81–90. - PubMed
-
- Michiels C. Endothelial cell functions. J Cell Physiol. 2003;196(3):430–43. - PubMed
-
- Nugent HM, Rogers C, Edelman ER. Endothelial implants inhibit intimal hyperplasia after porcine angioplasty. Circ Res. 1999;84(4):384–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

