PcpA of Streptococcus pneumoniae mediates adherence to nasopharyngeal and lung epithelial cells and elicits functional antibodies in humans
- PMID: 22796387
- PMCID: PMC3490615
- DOI: 10.1016/j.micinf.2012.06.007
PcpA of Streptococcus pneumoniae mediates adherence to nasopharyngeal and lung epithelial cells and elicits functional antibodies in humans
Abstract
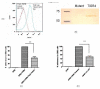
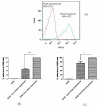
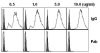
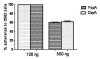
Streptococcus pneumoniae (pneumococci) adhere to human nasopharyngeal (NP) epithelial cells as a first step in colonization and adherence of pneumococci to lung epithelia may be required to establish pneumonia. We sought to determine if PcpA can serve as an adhesin to human NP (D562) and lung (A549) epithelial cells and whether PcpA mediated adherence can be inhibited by human anti-PcpA antibodies. A PcpA isogenic mutant was constructed in a pneumococcal TIGR4 background. When the mutant and wild type strains were compared for their adherence to D562 and A549 cell lines, a reduction in adherence by the mutant was observed (p = 0.0001 for both cell types). PcpA was ectopically expressed on the surface of minimally-adherent heterologous host Escherichia coli resulting in augmented adherence to D562 (p = 0.002) and A549 (p = 0.015) cells. Total IgG was purified from a pool of 6 human sera having high IgG titers of anti-pneumococcal proteins. The purified IgG reduced TIGR4 adherence to D562 cells but we determined that this effect was largely due to bacterial cell aggregation as determined by flow cytometry and confocal microscopy. Fab fragments were prepared from pooled IgG sera. Inhibition of TIGR4 adherence to D562 cells was observed using the Fab fragments without causing bacterial aggregation (p = 0.0001). Depletion of PcpA-specific Fab fragments resulted in an increase in adherence of TIGR4 to D562 cells (p = 0.028). We conclude that PcpA can mediate adherence of pneumococci to human NP and lung epithelial cells and PcpA mediated adherence can be inhibited by human anti-PcpA antibodies.
Copyright © 2012 Institut Pasteur. Published by Elsevier Masson SAS. All rights reserved.
Figures
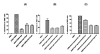
Similar articles
-
Vaccine candidates PhtD and PhtE of Streptococcus pneumoniae are adhesins that elicit functional antibodies in humans.Vaccine. 2012 Apr 16;30(18):2900-7. doi: 10.1016/j.vaccine.2012.02.023. Epub 2012 Feb 17. Vaccine. 2012. PMID: 22349524 Free PMC article.
-
Human antibodies to PhtD, PcpA, and Ply reduce adherence to human lung epithelial cells and murine nasopharyngeal colonization by Streptococcus pneumoniae.Infect Immun. 2014 Dec;82(12):5069-75. doi: 10.1128/IAI.02124-14. Epub 2014 Sep 22. Infect Immun. 2014. PMID: 25245804 Free PMC article.
-
Streptococcus pneumoniae surface protein PcpA elicits protection against lung infection and fatal sepsis.Infect Immun. 2008 Jun;76(6):2767-76. doi: 10.1128/IAI.01126-07. Epub 2008 Apr 7. Infect Immun. 2008. PMID: 18391008 Free PMC article.
-
Pneumococcal surface adhesin A (PsaA): a review.Crit Rev Microbiol. 2008;34(3-4):131-42. doi: 10.1080/10408410802275352. Crit Rev Microbiol. 2008. PMID: 18728990 Review.
-
The impact of the ancillary pilus-1 protein RrgA of Streptococcus pneumoniae on colonization and disease.Mol Microbiol. 2020 Mar;113(3):650-658. doi: 10.1111/mmi.14451. Mol Microbiol. 2020. PMID: 32185835 Review.
Cited by
-
Poor memory B cell generation contributes to non-protective responses to DTaP vaccine antigens in otitis-prone children.Clin Exp Immunol. 2015 Dec;182(3):314-22. doi: 10.1111/cei.12660. Epub 2015 Sep 30. Clin Exp Immunol. 2015. PMID: 26138025 Free PMC article. Clinical Trial.
-
The transcriptomic response of Streptococcus pneumoniae following exposure to cigarette smoke extract.Sci Rep. 2018 Oct 24;8(1):15716. doi: 10.1038/s41598-018-34103-5. Sci Rep. 2018. PMID: 30356075 Free PMC article.
-
The Two-Component System YesMN Promotes Pneumococcal Host-to-Host Transmission and Regulates Genes Involved in Zinc Homeostasis.Infect Immun. 2023 Jan 24;91(1):e0037522. doi: 10.1128/iai.00375-22. Epub 2022 Dec 20. Infect Immun. 2023. PMID: 36537790 Free PMC article.
-
State-of-the-art in the pneumococcal field: Proceedings of the 11th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-11).Pneumonia (Nathan). 2020 Feb 5;12:2. doi: 10.1186/s41479-019-0064-y. eCollection 2020. Pneumonia (Nathan). 2020. PMID: 32042572 Free PMC article. Review.
-
Streptococcus pneumoniae synchronizes the states of cell wall peptidoglycan acetylation and genome methylation by programmed DNA inversions.PLoS Pathog. 2025 Aug 5;21(8):e1013286. doi: 10.1371/journal.ppat.1013286. eCollection 2025 Aug. PLoS Pathog. 2025. PMID: 40763171 Free PMC article.
References
-
- Murphy TF, Yi K. Mechanisms of recurrent otitis media: importance of the immune response to bacterial surface antigens. Ann.N.Y.Acad.Sci. 1997;830:353–60. - PubMed
-
- Tuomanen EI, Austrian R, Masure HR. Pathogenesis of pneumococcal infection. N.Engl.J.Med. 1995;332:1280–4. - PubMed
-
- Jensch I, Gamez G, Rothe M, et al. PavB is a surface-exposed adhesin of Streptococcus pneumoniae contributing to nasopharyngeal colonization and airways infections. Mol.Microbiol. 2010;77:22–43. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

