Orexin A decreases lipid peroxidation and apoptosis in a novel hypothalamic cell model
- PMID: 22796468
- PMCID: PMC4467811
- DOI: 10.1016/j.neulet.2012.07.002
Orexin A decreases lipid peroxidation and apoptosis in a novel hypothalamic cell model
Abstract
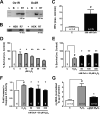
Current data support the idea that hypothalamic neuropeptide orexin A (OxA; hypocretin 1) mediates resistance to high fat diet-induced obesity. We previously demonstrated that OxA elevates spontaneous physical activity (SPA), that rodents with high SPA have higher endogenous orexin sensitivity, and that OxA-induced SPA contributes to obesity resistance in rodents. Recent reports show that OxA can confer neuroprotection against ischemic damage, and may decrease lipid peroxidation. This is noteworthy as independent lines of evidence indicate that diets high in saturated fats can decrease SPA, increase hypothalamic apoptosis, and lead to obesity. Together data suggest OxA may protect against obesity both by inducing SPA and by modulation of anti-apoptotic mechanisms. While OxA effects on SPA are well characterized, little is known about the short- and long-term effects of hypothalamic OxA signaling on intracellular neuronal metabolic status, or the physiological relevance of such signaling to SPA. To address this issue, we evaluated the neuroprotective effects of OxA in a novel immortalized primary embryonic rat hypothalamic cell line. We demonstrate for the first time that OxA increases cell viability during hydrogen peroxide challenge, decreases hydrogen peroxide-induced lipid peroxidative stress, and decreases caspase 3/7 induced apoptosis in an in vitro hypothalamic model. Our data support the hypothesis that OxA may promote obesity resistance both by increasing SPA, and by influencing survival of OxA-responsive hypothalamic neurons. Further identification of the individual mediators of the anti-apoptotic and peroxidative effects of OxA on target neurons could lead to therapies designed to maintain elevated SPA and increase obesity resistance.
Copyright © 2012 Elsevier Ireland Ltd. All rights reserved.
Figures
References
-
- Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Cell culture models of oxidative stress and injury in the central nervous system. Curr. Neurovasc. Res. 2005;2:73–89. - PubMed
-
- Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, Akerman KE, Kukkonen JP. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J. Pharmacol. Exp. Ther. 2003;305:507–514. - PubMed
-
- Ammoun S, Johansson L, Ekholm ME, Holmqvist T, Danis AS, Korhonen L, Sergeeva OA, Haas HL, Akerman KE, Kukkonen JP. OX1 orexin receptors activate extracellular signal-regulated kinase in Chinese hamster ovary cells via multiple mechanisms: the role of Ca2+ influx in OX1 receptor signaling. Mol. Endocrinol. 2006;20:80–99. - PubMed
-
- Belsham DD, Cai F, Cui H, Smukler SR, Salapatek AM, Shkreta L. Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology. 2004;145:393–400. - PubMed
-
- Benani A, Troy S, Carmona MC, Fioramonti X, Lorsignol A, Leloup C, Casteilla L, Penicaud L. Role for mitochondrial reactive oxygen species in brain lipid sensing: redox regulation of food intake. Diabetes. 2007;56:152–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

