Desmoglein 3 acting as an upstream regulator of Rho GTPases, Rac-1/Cdc42 in the regulation of actin organisation and dynamics
- PMID: 22796473
- PMCID: PMC4022105
- DOI: 10.1016/j.yexcr.2012.07.002
Desmoglein 3 acting as an upstream regulator of Rho GTPases, Rac-1/Cdc42 in the regulation of actin organisation and dynamics
Abstract
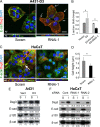
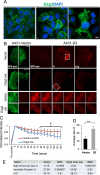
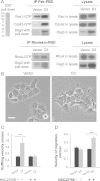
Desmoglein 3 (Dsg3), a member of the desmoglein sub-family, serves as an adhesion molecule in desmosomes. Our previous study showed that overexpression of human Dsg3 in several epithelial lines induces formation of membrane protrusions, a phenotype suggestive of Rho GTPase activation. Here we examined the interaction between Dsg3 and actin in detail and showed that endogenous Dsg3 colocalises and interacts with actin, particularly the junctional actin in a Rac1-dependent manner. Ablation of Rac1 activity by dominant negative Rac1 mutant (N17Rac1) or the Rac1 specific inhibitor (NSC23766) directly disrupts the interaction between Dsg3 and actin. Assembly of the junctional actin at the cell borders is accompanied with enhanced levels of Dsg3, while inhibition of Dsg3 by RNAi results in profound changes in the organisation of actin cytoskeleton. In accordance, overexpression of Dsg3 results in a remarkable increase of Rac1 and Cdc42 activities and to a lesser extent, RhoA. The enhancements in Rho GTPases are accompanied by the pronounced actin-based membrane structures such as lamellipodia and filopodia, enhanced rate of actin turnover and cell polarisation. Together, our results reveal an important novel function for Dsg3 in promoting actin dynamics through regulating Rac1 and Cdc42 activation in epithelial cells.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures







References
-
- Rudini N., Dejana E. Adherens junctions. Curr. Biol. 2008;18:R1080–R1082. - PubMed
-
- Etienne-Manneville S. Control of polarized cell morphology and motility by adherens junctions. Semin. Cell Dev. Biol. 2011;22:850–857. - PubMed
-
- Amagai M., Klaus-Kovtun V., Stanley J.R. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–877. - PubMed
-
- Amagai M., Ahmed A.R., Kitajima Y., Bystryn J.C., Milner Y., Gniadecki R., Hertl M., Pincelli C., Kurzen H., Fridkis-Hareli M., Aoyama Y., Frusic-Zlotkin M., Muller E., David M., Mimouni D., Vind-Kezunovic D., Michel B., Mahoney M., Grando S. Are desmoglein autoantibodies essential for the immunopathogenesis of pemphigus vulgaris, or just witnesses of disease? Exp. Dermatol. 2006;15:815–831. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

