Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation
- PMID: 22796535
- PMCID: PMC4320643
- DOI: 10.1016/j.bbmt.2012.07.003
Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation
Abstract
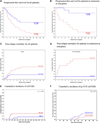
Haploidentical stem cell transplantation (SCT) has been generally performed using a T cell depleted (TCD) graft; however, a high rate of nonrelapse mortality (NRM) has been reported, particularly in adult patients. We hypothesized that using a T cell replete (TCR) graft followed by effective posttransplantation immunosuppressive therapy would reduce NRM and improve outcomes. We analyzed 65 consecutive adult patients with hematologic malignancies who received TCR (N = 32) or TCD (N = 33) haploidentical transplants. All patients received a preparative regimen consisting of melphalan, fludarabine, and thiotepa. The TCR group received posttransplantation treatment with cyclophosphamide (Cy), tacrolimus (Tac), and mycophenolate mofetil (MMF). Patients with TCD received antithymocyte globulin followed by infusion of CD34+ selected cells with no posttransplantation immunosuppression. The majority of patients in each group had active disease at the time of transplantation. Outcomes are reported for the TCR and TCD recipients, respectively. Engraftment was achieved in 94% versus 81% (P = NS). NRM at 1 year was 16% versus 42% (P = .02). Actuarial overall survival (OS) and progression-free survival (PFS) rates at 1 year posttransplantation were 64% versus 30% (P = .02) and 50% versus 21% (P = .02). The cumulative incidence of grade II-IV acute graft-versus-host disease (aGVHD) was 20% versus 11% (P = .20), and chronic GVHD (cGVHD) 7% versus 18% (P = .03). Improved reconstitution of T cell subsets and a lower rate of infection were observed in the TCR group. These results indicate that a TCR graft followed by effective control of GVHD posttransplantation may lower NRM and improve survival after haploidentical SCT.
Copyright © 2012 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Haploidentical transplantation: repurposing cyclophosphamide.Biol Blood Marrow Transplant. 2012 Dec;18(12):1771-2. doi: 10.1016/j.bbmt.2012.09.005. Epub 2012 Sep 27. Biol Blood Marrow Transplant. 2012. PMID: 23022466 No abstract available.
References
-
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. - PubMed
-
- Powles RL, Morgenstern GR, Kay HE, et al. Mismatched family donors for bone-marrow transplantation as treatment for acute leukemia. Lancet. 1983;1:612–615. - PubMed
-
- Marmont AM, Horowitz MM, Gale RP, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78:2120–2130. - PubMed
-
- Aversa F, Tabilio A, Terenzi A, et al. Successful engraftment of T-cell-depleted haploidentical “three-loci” incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood. 1994;84:3948–3955. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

