Distribution and frequency of intranuclear inclusions in female CGG KI mice modeling the fragile X premutation
- PMID: 22796595
- PMCID: PMC3572858
- DOI: 10.1016/j.brainres.2012.06.052
Distribution and frequency of intranuclear inclusions in female CGG KI mice modeling the fragile X premutation
Abstract
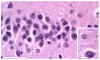
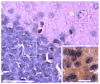
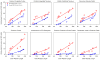
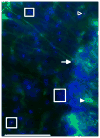
The fragile X-associated tremor/ataxia syndrome (FXTAS) is an adult-onset neurodegenerative disorder caused by CGG trinucleotide repeat expansions in the fragile X mental retardation 1 (FMR1) gene. The neuropathological hallmark of FXTAS is the presence of ubiquitin-positive intranuclear inclusions in neurons and in astroglia. Intranuclear inclusions have also been reported in the neurons of male CGG KI mice carrying an expanded CGG trinucleotide repeat and used to model FXTAS, but no study has been carried out quantifying inclusions in female CGG KI mice heterozygous for the fragile X premutation. We used histologic and immunocytochemical methods to determine the pathological features of intranuclear inclusions in astroglia and neurons. In female CGG KI mice, ubiquitin-positive intranuclear inclusions were found in neurons and astroglia throughout the brain in cortical and subcortical regions. These inclusions increased in number and became larger with advanced age and increasing CGG repeat length, supporting hypotheses that these pathologic features are progressive across the lifespan. The number of inclusions in neurons was reduced by ∼25% in female CGG KI mice compared to male CGG KI mice, but not so low as the 50% predicted. These data emphasize the need to evaluate the neurocognitive and pathological features in female carriers of the fragile X premutation with and without FXTAS symptomatology is warranted, as this population shows similar neuropathological features present in male FXTAS patients.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Astroglial-targeted expression of the fragile X CGG repeat premutation in mice yields RAN translation, motor deficits and possible evidence for cell-to-cell propagation of FXTAS pathology.Acta Neuropathol Commun. 2019 Feb 26;7(1):27. doi: 10.1186/s40478-019-0677-7. Acta Neuropathol Commun. 2019. PMID: 30808398 Free PMC article.
-
Ubiquitin-positive intranuclear inclusions in neuronal and glial cells in a mouse model of the fragile X premutation.Brain Res. 2010 Mar 8;1318:155-66. doi: 10.1016/j.brainres.2009.12.077. Epub 2010 Jan 4. Brain Res. 2010. PMID: 20051238 Free PMC article.
-
Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS).Brain. 2006 Jan;129(Pt 1):243-55. doi: 10.1093/brain/awh683. Epub 2005 Dec 5. Brain. 2006. PMID: 16332642
-
Mouse models of fragile X-associated tremor ataxia.J Investig Med. 2009 Dec;57(8):837-41. doi: 10.2310/JIM.0b013e3181af59d6. J Investig Med. 2009. PMID: 19574928 Free PMC article. Review.
-
Potential pathogenic mechanisms underlying Fragile X Tremor Ataxia Syndrome: RAN translation and/or RNA gain-of-function?Eur J Med Genet. 2018 Nov;61(11):674-679. doi: 10.1016/j.ejmg.2017.11.001. Epub 2017 Dec 6. Eur J Med Genet. 2018. PMID: 29223504 Review.
Cited by
-
Intranuclear inclusions in a fragile X mosaic male.Transl Neurodegener. 2013 May 21;2(1):10. doi: 10.1186/2047-9158-2-10. Transl Neurodegener. 2013. PMID: 23692864 Free PMC article.
-
Astroglial-targeted expression of the fragile X CGG repeat premutation in mice yields RAN translation, motor deficits and possible evidence for cell-to-cell propagation of FXTAS pathology.Acta Neuropathol Commun. 2019 Feb 26;7(1):27. doi: 10.1186/s40478-019-0677-7. Acta Neuropathol Commun. 2019. PMID: 30808398 Free PMC article.
-
Reduction of Fmr1 mRNA Levels Rescues Pathological Features in Cortical Neurons in a Model of FXTAS.Mol Ther Nucleic Acids. 2019 Dec 6;18:546-553. doi: 10.1016/j.omtn.2019.09.018. Epub 2019 Sep 26. Mol Ther Nucleic Acids. 2019. PMID: 31671347 Free PMC article.
-
Mouse models of the fragile X premutation and fragile X-associated tremor/ataxia syndrome.J Neurodev Disord. 2014;6(1):25. doi: 10.1186/1866-1955-6-25. Epub 2014 Jul 30. J Neurodev Disord. 2014. PMID: 25136376 Free PMC article. Review.
-
Neurocognitive endophenotypes in CGG KI and Fmr1 KO mouse models of Fragile X-Associated disorders: an analysis of the state of the field.F1000Res. 2013 Dec 27;2:287. doi: 10.12688/f1000research.2-287.v1. eCollection 2013. F1000Res. 2013. PMID: 24627796 Free PMC article. Review.
References
-
- Adams JS, Adams PE, Nguyen D, Brunberg JA, Tassone F, Zhang W, Koldewyn K, Rivera SM, Grigsby J, Zhang L, DeCarli C, Hagerman PJ, Hagerman RJ. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS) Neurology. 2007;69:851–859. - PubMed
-
- Al-Hinti JT, Nagan N, Harik SI. Fragile X premutation in a woman with cognitive impairment, tremor, and history of premature ovarian failure. Alzheimer Dis Assoc Disord. 2007;21:262–264. - PubMed
-
- Allen Mouse Brain Atlas [Internet] Seattle (WA): Allen Institute for Brain Science ©; 2009. Available from: 〈 http://mouse.brain-map.org〉.
-
- Berry-Kravis E, Potanos K, Weinberg D, Zhou L, Goetz CG. Fragile X-associated tremor/ataxia syndrome in sisters related to X-inactivation. Ann Neurol. 2005;57:144–147. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

