Activation of opioid μ-receptors, but not δ- or κ-receptors, switches pulmonary C-fiber-mediated rapid shallow breathing into an apnea in anesthetized rats
- PMID: 22796630
- PMCID: PMC3428499
- DOI: 10.1016/j.resp.2012.06.032
Activation of opioid μ-receptors, but not δ- or κ-receptors, switches pulmonary C-fiber-mediated rapid shallow breathing into an apnea in anesthetized rats
Abstract
Rapid shallow breathing (RSB) is mainly mediated by bronchopulmonary C-fibers (PCFs). We asked whether this RSB could be modulated by opioids. In anesthetized rats right atrial bolus injection of phenylbiguanide (PBG) to evoke RSB was repeated after: (1) intravenously giving fentanyl (μ-receptor agonist), DPDPE (δ-receptor agonist), or U-50488H (κ-receptor agonist); (2) fentanyl (iv) following naloxone methiodide, a peripheral opioid receptor antagonist; (3) bilateral microinjection of fentanyl into the nodose ganglia; (4) fentanyl (iv) with pre-blocking histamine H(1) and H(2) receptors by diphenhydramine and ranitidine. Systemic fentanyl challenge, but not DPDPE or U-50488H, switched the PBG-induced RSB to a long lasting apnea. This switch was blocked by naloxone methiodide rather than diphenhydramine and ranitidine. After microinjecting fentanyl into the nodose ganglia, PBG also produced an apnea. Our results suggest that activating μ-receptors is capable of turning the PCF-mediated RSB into an apnea, at least partly, via facilitating PCFs' activity and this switching effect appears independent of the released histamine.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures

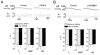
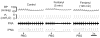



Similar articles
-
Contribution of central μ-receptors to switching pulmonary C-fibers-mediated rapid shallow breathing into an apnea by fentanyl in anesthetized rats.Brain Res. 2012 Aug 21;1469:73-81. doi: 10.1016/j.brainres.2012.06.028. Epub 2012 Jun 30. Brain Res. 2012. PMID: 22759907 Free PMC article.
-
8-OH-DPAT abolishes the pulmonary C-fiber-mediated apneic response to fentanyl largely via acting on 5HT1A receptors in the nucleus tractus solitarius.Am J Physiol Regul Integr Comp Physiol. 2012 Aug 15;303(4):R449-58. doi: 10.1152/ajpregu.00016.2012. Epub 2012 Jun 13. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22696579 Free PMC article.
-
Intravenous bolus injection of fentanyl triggers an immediate central and upper airway obstructive apnea via activating vagal sensory afferents.J Appl Physiol (1985). 2024 Dec 1;137(6):1666-1677. doi: 10.1152/japplphysiol.00614.2024. Epub 2024 Oct 17. J Appl Physiol (1985). 2024. PMID: 39417800
-
Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo.J Physiol. 2009 Jun 1;587(Pt 11):2677-92. doi: 10.1113/jphysiol.2009.171678. Epub 2009 Apr 29. J Physiol. 2009. PMID: 19403616 Free PMC article.
-
[The mu, delta and kappa properties of various opioids].Masui. 1995 Sep;44(9):1228-32. Masui. 1995. PMID: 8523655 Japanese.
Cited by
-
A method of nodose ganglia injection in Sprague-Dawley rat.J Vis Exp. 2014 Nov 25;(93):e52233. doi: 10.3791/52233. J Vis Exp. 2014. PMID: 25490160 Free PMC article.
-
Tropine exacerbates the ventilatory depressant actions of fentanyl in freely-moving rats.Front Pharmacol. 2024 Jun 24;15:1405461. doi: 10.3389/fphar.2024.1405461. eCollection 2024. Front Pharmacol. 2024. PMID: 38978984 Free PMC article.
-
Dynamic Rhythmogenic Network States Drive Differential Opioid Responses in the In Vitro Respiratory Network.J Neurosci. 2021 Dec 1;41(48):9919-9931. doi: 10.1523/JNEUROSCI.1329-21.2021. Epub 2021 Oct 25. J Neurosci. 2021. PMID: 34697095 Free PMC article.
-
Evidence for peripheral and central actions of codeine to dysregulate swallowing in the anesthetized cat.Front Neurol. 2024 Jun 13;15:1356603. doi: 10.3389/fneur.2024.1356603. eCollection 2024. Front Neurol. 2024. PMID: 38938779 Free PMC article.
-
Peripheral opioid receptor antagonism alleviates fentanyl-induced cardiorespiratory depression and is devoid of aversive behavior.Elife. 2025 Apr 1;13:RP104469. doi: 10.7554/eLife.104469. Elife. 2025. PMID: 40167443 Free PMC article.
References
-
- Barke KE, Hough LB. Opiates, mast cells and histamine release. Life Sci. 1993;53:1391–1399. - PubMed
-
- Braude S, Royston D, Coe C, Barnes PJ. Histamine increases lung permeability by an H2-receptor mechanism. Lancet. 1984;2:372–374. - PubMed
-
- Brookes ZL, Reilly CS, Lawton BK, Brown NJ. Intravenous anesthesia inhibits leukocyte-endothelial interactions and expression of CD11b after hemorrhage. Shock (Augusta, Ga. 2006;25:492–499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

