CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver
- PMID: 22796894
- PMCID: PMC3994510
- DOI: 10.1016/j.jhep.2012.07.008
CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver
Abstract
Background & aims: IL-17 secreting CD4 (Th17) and CD8 (Tc17) T cells have been implicated in immune-mediated liver diseases, but the molecular basis for their recruitment and positioning within the liver is unknown.
Methods: The phenotype and migratory behaviour of human liver-derived Th17 and Tc17 cells were investigated by flow cytometry and chemotaxis and flow-based adhesion assays. The recruitment of murine Th17 cells to the liver was studied in vivo using intra-vital microscopy.
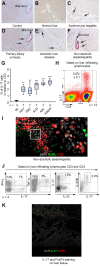
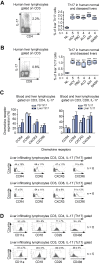
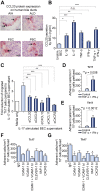
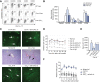
Results: IL-17(+) T cells comprised 1-3% of the T cell infiltrate in inflammatory liver diseases and included both CD4 (Th17) and CD8 (Tc17) cells. They expressed RORC and the IL-23 receptor and included subsets that secreted IL-22 and interferon-γ. Th17 and Tc17 cells expressed high levels of CXCR3 and CCR6, Tc17 cells also expressed CXCR6. Binding to human sinusoidal endothelium from flow was dependent on β1 and β2 integrins, CXCR3, and, in the case of Th17 cells, VAP-1. Th17 recruitment via sinusoids in mice with liver inflammation was reduced by treatment with antibodies against CXCR3 ligands, confirming the role of CXCR3 in Th17 recruitment in vivo. In human liver, IL-17(+) cells were detected in portal infiltrates close to inflamed bile ducts expressing the CCR6 ligand CCL20. Cytokine-treated human cholangiocytes secreted CCL20 and induced CCR6-dependent migration of Th17 cells suggesting that local cholangiocyte chemokine secretion localises Th17 cells to bile ducts.
Conclusions: CXCR3 promotes recruitment of Th17 cells from the blood into the liver in both human and murine liver injury. Their subsequent positioning near bile ducts is dependent on cholangiocyte-secreted CCL20.
Copyright © 2012. Published by Elsevier B.V.
Figures


Comment in
-
Th17 cells bound for the liver: group tourists or private travellers?J Hepatol. 2012 Nov;57(5):943-5. doi: 10.1016/j.jhep.2012.08.010. Epub 2012 Aug 16. J Hepatol. 2012. PMID: 22902346 No abstract available.
References
-
- Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. - PubMed
-
- Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. - PubMed
-
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. - PubMed
-
- Unutmaz D. RORC2: the master of human Th17 cell programming. Eur J Immunol. 2009;39:1452–1455. - PubMed
-
- Veldhoen M., Uyttenhove C., van S.J., Helmby H., Westendorf A., Buer J., et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

