The regulation of cell-cell adhesion during epithelial-mesenchymal transition, motility and tumor progression
- PMID: 22796940
- PMCID: PMC3478259
- DOI: 10.4161/cam.21326
The regulation of cell-cell adhesion during epithelial-mesenchymal transition, motility and tumor progression
Abstract
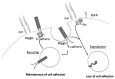
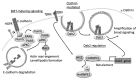
Adherens junctions (AJs) are essential for the maintenance of epithelial homeostasis and a key factor in the regulation of cell migration and tumor progression. AJs maintain cell-cell adhesion by linking transmembrane proteins to the actin cytoskeleton. Additionally, they participate in recruitment of signaling receptors and cytoplasmic proteins to the membrane. During cellular invasion or migration, AJs are dynamically regulated and their composition modified to initiate changes in signaling pathways and cytoskeleton organization involved in cellular motility. Loss of E-cadherin, a key component of AJs, is characteristic of epithelial-mesenchymal-transition (EMT) and is associated with tumor cell invasion. We will review recent findings describing novel mechanisms involved in E-cadherin transcription regulation, endocytosis of E-cadherin and signaling associated with loss of AJs as well as reorganization of the AJ during EMT.
Figures

References
-
- Chervin-Pétinot A, Courçon M, Almagro S, Nicolas A, Grichine A, Grunwald D, et al. Epithelial protein lost in neoplasm (EPLIN) interacts with α-catenin and actin filaments in endothelial cells and stabilizes vascular capillary network in vitro. J Biol Chem. 2012;287:7556–72. doi: 10.1074/jbc.M111.328682. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
