Effect of risedronate on bone in renal transplant recipients
- PMID: 22797188
- PMCID: PMC3402282
- DOI: 10.1681/ASN.2011060623
Effect of risedronate on bone in renal transplant recipients
Abstract
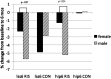
Bisphosphonates may prevent or treat the bone loss promoted by the immunosuppressive regimens used in renal transplantation. Risedronate is a commonly used third-generation amino-bisphosphonate, but little is known about its effects on the bone health of renal transplant recipients. We randomly assigned 42 new living-donor kidney recipients to either 35 mg of risedronate weekly or placebo for 12 months. We obtained bone biopsies at the time of renal transplant and after 12 months of protocol treatment. Treatment with risedronate did not affect bone mineral density (BMD) in the overall cohort. In subgroup analyses, it tended to preserve BMD in female participants but did not significantly affect the BMD of male participants. Risedronate did associate with increased osteoid volume and trabecular thickness in male participants, however. There was no evidence for the development of adynamic bone disease. In summary, further study is needed before the use of prophylactic bisphosphonates to attenuate bone loss can be recommended in renal transplant recipients.
Figures


References
-
- National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group: KDIGO clinical practice guideline for the diagnosis, evaluation, prevention and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int 76: S121–S130, 2009 - PubMed
-
- Coco M, Glicklich D, Faugere MC, Burris L, Bognar I, Durkin P, Tellis V, Greenstein S, Schechner R, Figueroa K, McDonough P, Wang G, Malluche H: Prevention of bone loss in renal transplant recipients: A prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol 14: 2669–2676, 2003 - PubMed
-
- Grotz W, Nagel C, Poeschel D, Cybulla M, Petersen KG, Uhl M, Strey C, Kirste G, Olschewski M, Reichelt A, Rump LC: Effect of ibandronate on bone loss and renal function after kidney transplantation. J Am Soc Nephrol 12: 1530–1537, 2001 - PubMed
-
- Haas M, Leko-Mohr Z, Roschger P, Kletzmayr J, Schwarz C, Mitterbauer C, Steininger R, Grampp S, Klaushofer K, Delling G, Oberbauer R: Zoledronic acid to prevent bone loss in the first 6 months after renal transplantation. Kidney Int 63: 1130–1136, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

