Metal imaging in neurodegenerative diseases
- PMID: 22797194
- PMCID: PMC4140182
- DOI: 10.1039/c2mt20052j
Metal imaging in neurodegenerative diseases
Abstract
Metal ions are known to play an important role in many neurodegenerative diseases including Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), and prion diseases. In these diseases, aberrant metal binding or improper regulation of redox active metal ions can induce oxidative stress by producing cytotoxic reactive oxygen species (ROS). Altered metal homeostasis is also frequently seen in the diseased state. As a result, the imaging of metals in intact biological cells and tissues has been very important for understanding the role of metals in neurodegenerative diseases. A wide range of imaging techniques have been utilized, including X-ray fluorescence microscopy (XFM), particle induced X-ray emission (PIXE), energy dispersive X-ray spectroscopy (EDS), laser ablation inductively coupled mass spectrometry (LA-ICP-MS), and secondary ion mass spectrometry (SIMS), all of which allow for the imaging of metals in biological specimens with high spatial resolution and detection sensitivity. These techniques represent unique tools for advancing the understanding of the disease mechanisms and for identifying possible targets for developing treatments. In this review, we will highlight the advances in neurodegenerative disease research facilitated by metal imaging techniques.
Figures
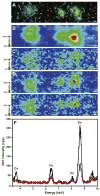


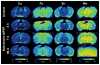
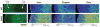
Similar articles
-
Metal and complementary molecular bioimaging in Alzheimer's disease.Front Aging Neurosci. 2014 Jul 15;6:138. doi: 10.3389/fnagi.2014.00138. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25076902 Free PMC article. Review.
-
Imaging of metals, metalloids, and non-metals by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) in biological tissues.Methods Mol Biol. 2010;656:51-82. doi: 10.1007/978-1-60761-746-4_3. Methods Mol Biol. 2010. PMID: 20680584
-
Comparative Study of Metal Quantification in Neurological Tissue Using Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry Imaging and X-ray Fluorescence Microscopy.Anal Chem. 2015 Jul 7;87(13):6639-45. doi: 10.1021/acs.analchem.5b01454. Epub 2015 Jun 9. Anal Chem. 2015. PMID: 26020362
-
Bioimaging of metals by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS).Mass Spectrom Rev. 2010 Jan-Feb;29(1):156-75. doi: 10.1002/mas.20239. Mass Spectrom Rev. 2010. PMID: 19557838 Review.
-
Bioimaging Metallomics.Adv Exp Med Biol. 2018;1055:139-181. doi: 10.1007/978-3-319-90143-5_7. Adv Exp Med Biol. 2018. PMID: 29884965 Review.
Cited by
-
Reducing Frost during Cryoimaging Using a Hygroscopic Ice Frame.ACS Omega. 2022 Nov 22;7(48):43421-43431. doi: 10.1021/acsomega.2c03083. eCollection 2022 Dec 6. ACS Omega. 2022. PMID: 36506191 Free PMC article.
-
In situ biospectroscopic investigation of rapid ischemic and postmortem induced biochemical alterations in the rat brain.ACS Chem Neurosci. 2015 Feb 18;6(2):226-38. doi: 10.1021/cn500157j. Epub 2014 Nov 13. ACS Chem Neurosci. 2015. PMID: 25350866 Free PMC article.
-
Reactivity of diphenylpropynone derivatives toward metal-associated amyloid-β species.Inorg Chem. 2012 Dec 3;51(23):12959-67. doi: 10.1021/ic302084g. Epub 2012 Nov 15. Inorg Chem. 2012. PMID: 23153071 Free PMC article.
-
Analytical Methods for Imaging Metals in Biology: From Transition Metal Metabolism to Transition Metal Signaling.Anal Chem. 2017 Jan 3;89(1):22-41. doi: 10.1021/acs.analchem.6b04631. Epub 2016 Dec 15. Anal Chem. 2017. PMID: 27976855 Free PMC article. Review. No abstract available.
-
Implications of ALS-Associated Mutations on Biochemical and Biophysical Features of hSOD1 and Aggregation Formation.Biochem Genet. 2024 Oct;62(5):3658-3680. doi: 10.1007/s10528-023-10619-y. Epub 2024 Jan 9. Biochem Genet. 2024. PMID: 38196030
References
-
- Taylor JP, Hardy J, Fischbeck KH. Toxic Proteins in Neurodegenerative Disease. Science. 2002;296:1991–1995. - PubMed
-
- Sayre LM, Smith MA, Perry G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Current Medicinal Chemistry. 2001;8:721–738. - PubMed
-
- Barnham KJ, Bush AI. Metals in Alzheimer’s and Parkinson’s Diseases. Curr Opin Chem Biol. 2008;12:222–228. - PubMed
-
- Lobinski R, Moulin C, Ortega R. Imaging and speciation of trace elements in biological environment. Biochimie. 2006;88:1591–1604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

