Viral acute lower respiratory infections impair CD8+ T cells through PD-1
- PMID: 22797302
- PMCID: PMC3408742
- DOI: 10.1172/JCI62860
Viral acute lower respiratory infections impair CD8+ T cells through PD-1
Abstract
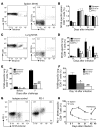
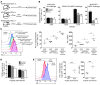
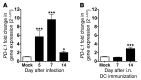
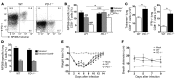
Viruses are leading causes of severe acute lower respiratory infections (LRIs). These infections evoke incomplete immunity, as individuals can be repeatedly reinfected throughout life. We report that acute viral LRI causes rapid pulmonary CD8+ cytotoxic T lymphocyte (TCD8) functional impairment via programmed death-1/programmed death ligand-1 (PD-1/PD-L1) signaling, a pathway previously associated with prolonged antigenic stimulation during chronic infections and cancer. PD-1-mediated TCD8 impairment occurred acutely in mice following infection with human metapneumovirus or influenza virus. Viral antigen was sufficient for PD-1 upregulation, but induction of PD-L1 was required for impairment. During secondary viral infection or epitope-only challenge, memory TCD8 rapidly reexpressed PD-1 and exhibited severe functional impairment. Inhibition of PD-1 signaling using monoclonal antibody blockade prevented TCD8 impairment, reduced viral titers during primary infection, and enhanced protection of immunized mice against challenge infection. Additionally, PD-1 and PD-L1 were upregulated in the lungs of patients with 2009 H1N1 influenza virus, respiratory syncytial virus, or parainfluenza virus infection. These results indicate that PD-1 mediates TCD8 functional impairment during acute viral infection and may contribute to recurrent viral LRIs. Therefore, the PD-1/PD-L1 pathway may represent a therapeutic target in the treatment of respiratory viruses.
Figures






References
-
- Langley GF, Anderson LJ. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr Infect Dis J. 2011;30(6):510–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 AI040079/AI/NIAID NIH HHS/United States
- R01 AI085062/AI/NIAID NIH HHS/United States
- P30 CA68485/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- KL2 RR024977/RR/NCRR NIH HHS/United States
- R21 AI082417/AI/NIAID NIH HHS/United States
- R01 HL054977/HL/NHLBI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- AI085062/AI/NIAID NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- HL054977/HL/NHLBI NIH HHS/United States
- AI082417/AI/NIAID NIH HHS/United States
- AI042286/AI/NIAID NIH HHS/United States
- TL1 RR024978/RR/NCRR NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- GM007347/GM/NIGMS NIH HHS/United States
- DK058404/DK/NIDDK NIH HHS/United States
- UL1 RR024975-01/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

