Simultaneous siRNA-mediated knockdown of antiapoptotic BCL2, Bcl-xL, XIAP and survivin in bladder cancer cells
- PMID: 22797576
- PMCID: PMC3583635
- DOI: 10.3892/ijo.2012.1549
Simultaneous siRNA-mediated knockdown of antiapoptotic BCL2, Bcl-xL, XIAP and survivin in bladder cancer cells
Abstract
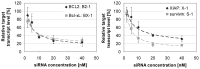
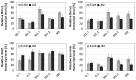
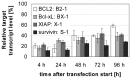
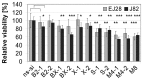
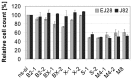
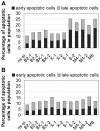
Bladder cancer (BCa) represents the ninth most common malignancy worldwide. Despite intensive treatment with surgery and chemotherapy the prognosis for BCa patients particularly at advanced stages is poor. The ability to evade apoptosis is a hallmark of cancer cells. Since the antiapoptotic genes BCL2, Bcl-xL, XIAP and survivin are frequently upregulated in BCa tissues, their combined siRNA-mediated knockdown might be more potent in decreasing BCa growth than the single inhibition of one target. Against each target two siRNAs were selected that specifically reduced the mRNA and protein levels of their appropriate target in EJ28 and J82 BCa cells. Inhibition of survivin provoked the strongest antiproliferative effect of all single target treatments, for example cell counts decreased by 50%. Simultaneous targeting of all four antiapoptotic genes downregulated expression levels of all targets and mediated significant reductions in cell viability and cell counts as well as induction of apoptosis. In EJ28 cells, combined knockdown of BCL2, Bcl-xL, XIAP and survivin caused a 2.5-fold enhancement in apoptosis rate and reduced cellular viability by 40%. Therefore, simultaneous knockdown of antiapoptotic BCL2, Bcl‑xL, XIAP and survivin may represent a promising treatment option for bladder cancer.
Figures

References
-
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. - PubMed
-
- Herr HW, Dotan Z, Donat SM, Bajorin DF. Defining optimal therapy for muscle invasive bladder cancer. J Urol. 2007;177:437–443. - PubMed
-
- Dogliotti L, Carteni G, Siena S, et al. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: results of a randomized phase 2 trial. Eur Urol. 2007;52:134–141. - PubMed
-
- Burz C, Berindan-Neagoe I, Balacescu O, Irimie A. Apoptosis in cancer: key molecular signaling pathways and therapy targets. Acta Oncol. 2009;48:811–821. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

