Dissecting spatial knowledge from spatial choice by hippocampal NMDA receptor deletion
- PMID: 22797694
- PMCID: PMC3442238
- DOI: 10.1038/nn.3166
Dissecting spatial knowledge from spatial choice by hippocampal NMDA receptor deletion
Abstract
Hippocampal NMDA receptors (NMDARs) and NMDAR-dependent synaptic plasticity are widely considered crucial substrates of long-term spatial memory, although their precise role remains uncertain. Here we show that Grin1(ΔDGCA1) mice, lacking GluN1 and hence NMDARs in all dentate gyrus and dorsal CA1 principal cells, acquired the spatial reference memory water maze task as well as controls, despite impairments on the spatial reference memory radial maze task. When we ran a spatial discrimination water maze task using two visually identical beacons, Grin1(ΔDGCA1) mice were impaired at using spatial information to inhibit selecting the decoy beacon, despite knowing the platform's actual spatial location. This failure could suffice to impair radial maze performance despite spatial memory itself being normal. Thus, these hippocampal NMDARs are not essential for encoding or storing long-term, associative spatial memories. Instead, we demonstrate an important function of the hippocampus in using spatial knowledge to select between alternative responses that arise from competing or overlapping memories.
Figures

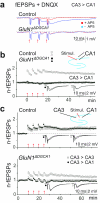



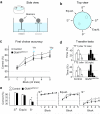

Comment in
-
Navigating uncertain waters.Nat Neurosci. 2012 Jul 26;15(8):1056-7. doi: 10.1038/nn.3174. Nat Neurosci. 2012. PMID: 22837031
References
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. - PubMed
-
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 2000;23:649–711. - PubMed
-
- Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat. Rev. Neurosci. 2008;9:65–75. - PubMed
-
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. - PubMed
-
- Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RG. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

