Beyond annexin V: fluorescence response of cellular membranes to apoptosis
- PMID: 22797774
- PMCID: PMC3560882
- DOI: 10.1007/s10616-012-9481-y
Beyond annexin V: fluorescence response of cellular membranes to apoptosis
Abstract
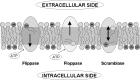
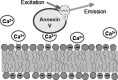
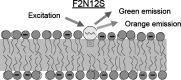
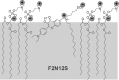
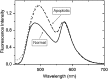
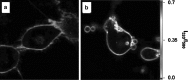
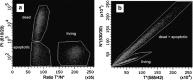
Dramatic changes in the structure of cell membranes on apoptosis allow easy, sensitive and non-destructive analysis of this process with the application of fluorescence methods. The strong plasma membrane asymmetry is present in living cells, and its loss on apoptosis is commonly detected with the probes interacting strongly and specifically with phosphatidylserine (PS). This phospholipid becomes exposed to the cell surface, and the application of annexin V labeled with fluorescent dye is presently the most popular tool for its detection. Several methods have been suggested recently that offer important advantages over annexin V assay with the ability to study apoptosis by spectroscopy of cell suspensions, flow cytometry and confocal or two-photon microscopy. The PS exposure marks the integrated changes in the outer leaflet of cell membrane that involve electrostatic potential and hydration, and the attempts are being made to provide direct probing of these changes. This review describes the basic mechanisms underlying the loss of membrane asymmetry during apoptosis and discusses, in comparison with the annexin V-binding assay, the novel fluorescence techniques of detecting apoptosis on cellular membrane level. In more detail we describe the detection method based on smart fluorescent dye F2N12S incorporated into outer leaflet of cell membrane and reporting on apoptotic cell transformation by easily detectable change of the spectral distribution of fluorescent emission. It can be adapted to any assay format.
Figures


References
LinkOut - more resources
Full Text Sources

