Mechanical elasticity as a physical signature of conformational dynamics in a virus particle
- PMID: 22797893
- PMCID: PMC3409779
- DOI: 10.1073/pnas.1207437109
Mechanical elasticity as a physical signature of conformational dynamics in a virus particle
Abstract
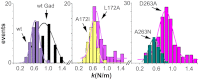
In this study we test the hypothesis that mechanically elastic regions in a virus particle (or large biomolecular complex) must coincide with conformationally dynamic regions, because both properties are intrinsically correlated. Hypothesis-derived predictions were subjected to verification by using 19 variants of the minute virus of mice capsid. The structural modifications in these variants reduced, preserved, or restored the conformational dynamism of regions surrounding capsid pores that are involved in molecular translocation events required for virus infectivity. The mechanical elasticity of the modified capsids was analyzed by atomic force microscopy, and the results corroborated every prediction tested: Any mutation (or chemical cross-linking) that impaired a conformational rearrangement of the pore regions increased their mechanical stiffness. On the contrary, any mutation that preserved the dynamics of the pore regions also preserved their elasticity. Moreover, any pseudo-reversion that restored the dynamics of the pore regions (lost through previous mutation) also restored their elasticity. Finally, no correlation was observed between dynamics of the pore regions and mechanical elasticity of other capsid regions. This study (i) corroborates the hypothesis that local mechanical elasticity and conformational dynamics in a viral particle are intrinsically correlated; (ii) proposes that determination by atomic force microscopy of local mechanical elasticity, combined with mutational analysis, may be used to identify and study conformationally dynamic regions in virus particles and large biomolecular complexes; (iii) supports a connection between mechanical properties and biological function in a virus; (iv) shows that viral capsids can be greatly stiffened by protein engineering for nanotechnological applications.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Structural basis for biologically relevant mechanical stiffening of a virus capsid by cavity-creating or spacefilling mutations.Sci Rep. 2017 Jun 22;7(1):4101. doi: 10.1038/s41598-017-04345-w. Sci Rep. 2017. PMID: 28642465 Free PMC article.
-
Quantitatively probing propensity for structural transitions in engineered virus nanoparticles by single-molecule mechanical analysis.Nanoscale. 2015 Mar 19;7(13):5654-64. doi: 10.1039/c4nr07046a. Nanoscale. 2015. PMID: 25744136
-
Fluctuating Nonlinear Spring Model of Mechanical Deformation of Biological Particles.PLoS Comput Biol. 2016 Jan 28;12(1):e1004729. doi: 10.1371/journal.pcbi.1004729. eCollection 2016 Jan. PLoS Comput Biol. 2016. PMID: 26821264 Free PMC article.
-
Mechanical Properties of Viruses.Subcell Biochem. 2024;105:629-691. doi: 10.1007/978-3-031-65187-8_18. Subcell Biochem. 2024. PMID: 39738960 Review.
-
Atomic force microscopy observation and characterization of single virions and virus-like particles by nano-indentation.Curr Opin Virol. 2016 Jun;18:82-8. doi: 10.1016/j.coviro.2016.05.002. Epub 2016 May 30. Curr Opin Virol. 2016. PMID: 27253691 Review.
Cited by
-
Synonymous mutations reduce genome compactness in icosahedral ssRNA viruses.Biophys J. 2015 Jan 6;108(1):194-202. doi: 10.1016/j.bpj.2014.10.070. Biophys J. 2015. PMID: 25564866 Free PMC article.
-
Single-particle virology.Biophys Rev. 2020 Oct;12(5):1141-1154. doi: 10.1007/s12551-020-00747-9. Epub 2020 Sep 3. Biophys Rev. 2020. PMID: 32880826 Free PMC article. Review.
-
Mechanical and assembly units of viral capsids identified via quasi-rigid domain decomposition.PLoS Comput Biol. 2013;9(11):e1003331. doi: 10.1371/journal.pcbi.1003331. Epub 2013 Nov 14. PLoS Comput Biol. 2013. PMID: 24244139 Free PMC article.
-
Fluctuating nonlinear spring theory: Strength, deformability, and toughness of biological nanoparticles from theoretical reconstruction of force-deformation spectra.Acta Biomater. 2021 Mar 1;122:263-277. doi: 10.1016/j.actbio.2020.12.043. Epub 2020 Dec 28. Acta Biomater. 2021. PMID: 33359294 Free PMC article.
-
Systematic analysis of biological roles of charged amino acid residues located throughout the structured inner wall of a virus capsid.Sci Rep. 2018 Jun 22;8(1):9543. doi: 10.1038/s41598-018-27749-8. Sci Rep. 2018. PMID: 29934575 Free PMC article.
References
-
- Chiu W, Burnett RM, Garcea RL, editors. Structural Biology of Viruses. New York: Oxford Univ Press; 1997.
-
- Agbandje-McKenna M, McKenna R, editors. Structural Virology. Cambridge, UK: RSC Publishing; 2011.
-
- Johnson JE. Virus particle dynamics. Adv Protein Chem. 2003;64:197–218. - PubMed
-
- Bothner B, et al. Crystallographically identical virus capsids display different properties in solution. Nat Struct Biol. 1999;6:114–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

