The tyrosine phosphatase SHP-1 negatively regulates cytotrophoblast proliferation in first-trimester human placenta by modulating EGFR activation
- PMID: 22797910
- PMCID: PMC11115170
- DOI: 10.1007/s00018-012-1067-5
The tyrosine phosphatase SHP-1 negatively regulates cytotrophoblast proliferation in first-trimester human placenta by modulating EGFR activation
Abstract
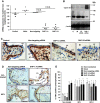
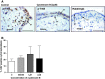
Insulin-like growth factors (IGFs) influence placental cell (cytotrophoblast) kinetics. We recently reported that the protein tyrosine phosphatase (PTP) SHP-2 positively regulates IGF actions in the placenta. In other systems, the closely related PTP, SHP-1, functions as a negative regulator of signaling events but its role in the placenta is still unknown. We examined the hypothesis that SHP-1 negatively regulates IGF actions in the human placenta. Immunohistochemical (IHC) analysis demonstrated that SHP-1 is abundant in cytotrophoblast. SHP-1 expression was decreased in first-trimester placental explants using siRNA; knockdown did not alter IGF-induced proliferation but it significantly enhanced proliferation in serum-free conditions, revealing that placental growth is endogenously regulated. Candidate regulators were determined by using antibody arrays, Western blotting, and IHC to examine the activation status of multiple receptor tyrosine kinases (RTKs) in SHP-1-depleted explants; amongst the alterations observed was enhanced activation of EGFR, suggesting that SHP-1 may interact with EGFR to inhibit proliferation. The EGFR tyrosine kinase inhibitor PD153035 reversed the elevated proliferation seen in the absence of SHP-1. This study demonstrates a role for SHP-1 in human trophoblast turnover and establishes SHP-1 as a negative regulator of EGFR activation. Targeting placental SHP-1 expression may provide therapeutic benefits in common pregnancy conditions with abnormal trophoblast proliferation.
Figures



Similar articles
-
MicroRNA regulation of mitogenic signaling networks in the human placenta.J Biol Chem. 2014 Oct 31;289(44):30404-30416. doi: 10.1074/jbc.M114.587295. Epub 2014 Jul 30. J Biol Chem. 2014. PMID: 25077964 Free PMC article.
-
The protein-tyrosine phosphatase, SRC homology-2 domain containing protein tyrosine phosphatase-2, is a crucial mediator of exogenous insulin-like growth factor signaling to human trophoblast.Endocrinology. 2009 Oct;150(10):4744-54. doi: 10.1210/en.2009-0166. Epub 2009 Jul 9. Endocrinology. 2009. PMID: 19589868
-
miR-514a-3p: a novel SHP-2 regulatory miRNA that modulates human cytotrophoblast proliferation.J Mol Endocrinol. 2022 Jan 20;68(2):99-110. doi: 10.1530/JME-21-0175. J Mol Endocrinol. 2022. PMID: 34792485 Free PMC article.
-
Insulin-like growth factor I and II regulate the life cycle of trophoblast in the developing human placenta.Am J Physiol Cell Physiol. 2008 Jun;294(6):C1313-22. doi: 10.1152/ajpcell.00035.2008. Epub 2008 Apr 9. Am J Physiol Cell Physiol. 2008. PMID: 18400990
-
Transforming growth factor-{beta} (TGF{beta}) receptors I/II differentially regulate TGF{beta}1 and IGF-binding protein-3 mitogenic effects in the human placenta.Endocrinology. 2010 Apr;151(4):1723-31. doi: 10.1210/en.2009-0896. Epub 2010 Feb 19. Endocrinology. 2010. PMID: 20172969
Cited by
-
Statins inhibit insulin-like growth factor action in first trimester placenta by altering insulin-like growth factor 1 receptor glycosylation.Mol Hum Reprod. 2015 Jan;21(1):105-14. doi: 10.1093/molehr/gau093. Epub 2014 Oct 9. Mol Hum Reprod. 2015. PMID: 25304981 Free PMC article.
-
METTL14 benefits the mesenchymal stem cells in patients with steroid-associated osteonecrosis of the femoral head by regulating the m6A level of PTPN6.Aging (Albany NY). 2021 Dec 15;13(24):25903-25919. doi: 10.18632/aging.203778. Epub 2021 Dec 15. Aging (Albany NY). 2021. PMID: 34910686 Free PMC article.
-
Protein tyrosine phosphatase SHP-1 sensitizes EGFR/HER-2 positive breast cancer cells to trastuzumab through modulating phosphorylation of EGFR and HER-2.Onco Targets Ther. 2015 Sep 14;8:2577-87. doi: 10.2147/OTT.S82225. eCollection 2015. Onco Targets Ther. 2015. PMID: 26396531 Free PMC article.
-
Siglec-6 Signaling Uses Src Kinase Tyrosine Phosphorylation and SHP-2 Recruitment.Cells. 2022 Oct 29;11(21):3427. doi: 10.3390/cells11213427. Cells. 2022. PMID: 36359823 Free PMC article.
-
MicroRNA regulation of mitogenic signaling networks in the human placenta.J Biol Chem. 2014 Oct 31;289(44):30404-30416. doi: 10.1074/jbc.M114.587295. Epub 2014 Jul 30. J Biol Chem. 2014. PMID: 25077964 Free PMC article.
References
-
- Forbes K, West G, Garside R, Aplin JD, Westwood M. The protein-tyrosine phosphatase, SRC homology-2 domain containing protein tyrosine phosphatase-2, is a crucial mediator of exogenous insulin-like growth factor signaling to human trophoblast. Endocrinology. 2009;150:4744–4754. doi: 10.1210/en.2009-0166. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

