United we stand: integrating the actin cytoskeleton and cell-matrix adhesions in cellular mechanotransduction
- PMID: 22797913
- PMCID: PMC3434863
- DOI: 10.1242/jcs.093716
United we stand: integrating the actin cytoskeleton and cell-matrix adhesions in cellular mechanotransduction
Abstract
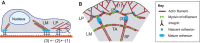
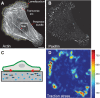
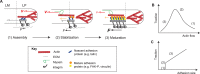
Many essential cellular functions in health and disease are closely linked to the ability of cells to respond to mechanical forces. In the context of cell adhesion to the extracellular matrix, the forces that are generated within the actin cytoskeleton and transmitted through integrin-based focal adhesions are essential for the cellular response to environmental clues, such as the spatial distribution of adhesive ligands or matrix stiffness. Whereas substantial progress has been made in identifying mechanosensitive molecules that can transduce mechanical force into biochemical signals, much less is known about the nature of cytoskeletal force generation and transmission that regulates the magnitude, duration and spatial distribution of forces imposed on these mechanosensitive complexes. By focusing on cell-matrix adhesion to flat elastic substrates, on which traction forces can be measured with high temporal and spatial resolution, we discuss our current understanding of the physical mechanisms that integrate a large range of molecular mechanotransduction events on cellular scales. Physical limits of stability emerge as one important element of the cellular response that complements the structural changes affected by regulatory systems in response to mechanical processes.
Figures

References
-
- Alexandrova A. Y., Arnold K., Schaub S., Vasiliev J. M., Meister J-J., Bershadsky A. D., Verkhovsky A. B. (2008). Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE 3, e3234 10.1371/journal.pone.0003234 - DOI - PMC - PubMed
-
- Balaban N. Q., Schwarz U. S., Riveline D., Goichberg P., Tzur G., Sabanay I., Mahalu D., Safran S., Bershadsky A., Addadi L.et al. (2001). Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3, 466–472 10.1038/35074532 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

