MLL5 maintains genomic integrity by regulating the stability of the chromosomal passenger complex through a functional interaction with Borealin
- PMID: 22797924
- PMCID: PMC3500868
- DOI: 10.1242/jcs.110411
MLL5 maintains genomic integrity by regulating the stability of the chromosomal passenger complex through a functional interaction with Borealin
Abstract
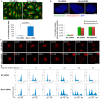
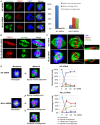
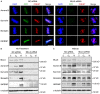
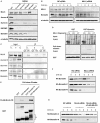
Mixed lineage leukemia 5 (MLL5) is a versatile nuclear protein associated with many cellular events. We have shown previously that phosphorylation of MLL5 by Cdk1 is required for mitotic entry. In this paper, the function of MLL5 in mitotic regulation is further explored. SiRNA-mediated downregulation of MLL5 caused improper chromosome alignment at metaphase and resulted in failure of DNA segregation and cytokinesis. Mechanistic studies revealed that the chromosomal passenger complex (CPC), which plays a key role in chromosomal bi-orientation, was delocalized from the inner centromere region because of proteasome-mediated degradation in MLL5-depleted cells. Biochemical analyses further demonstrated that the central domain of MLL5 interacted with the C-terminus of Borealin, and the interaction is essential to maintain the stability of Borealin. Moreover, the mitotic defects in MLL5-depleted cells were rescued by overexpression of FLAG-MLL5, but not by a FLAG-MLL5 mutant that did not contain the central domain. Collectively, our results suggest that MLL5 functionally interacts with Borealin, facilitates the expression of CPC, and hence contributes to mitotic fidelity and genomic integrity.
Figures
Similar articles
-
The inner centromere is a biomolecular condensate scaffolded by the chromosomal passenger complex.Nat Cell Biol. 2019 Sep;21(9):1127-1137. doi: 10.1038/s41556-019-0376-4. Epub 2019 Sep 3. Nat Cell Biol. 2019. PMID: 31481798 Free PMC article.
-
Australin: a chromosomal passenger protein required specifically for Drosophila melanogaster male meiosis.J Cell Biol. 2008 Feb 11;180(3):521-35. doi: 10.1083/jcb.200708072. J Cell Biol. 2008. PMID: 18268101 Free PMC article.
-
The Borealin dimerization domain interacts with Sgo1 to drive Aurora B-mediated spindle assembly.Mol Biol Cell. 2020 Sep 15;31(20):2207-2218. doi: 10.1091/mbc.E20-05-0341. Epub 2020 Jul 22. Mol Biol Cell. 2020. PMID: 32697622 Free PMC article.
-
Cell division control by the Chromosomal Passenger Complex.Exp Cell Res. 2012 Jul 15;318(12):1407-20. doi: 10.1016/j.yexcr.2012.03.015. Epub 2012 Mar 24. Exp Cell Res. 2012. PMID: 22472345 Review.
-
Chromosomal passengers: the four-dimensional regulation of mitotic events.Chromosoma. 2004 Nov;113(5):211-22. doi: 10.1007/s00412-004-0307-3. Epub 2004 Sep 4. Chromosoma. 2004. PMID: 15351889 Review.
Cited by
-
Case Report: De novo Variants of KMT2E Cause O'Donnell-Luria-Rodan Syndrome: Additional Cases and Literature Review.Front Pediatr. 2021 Feb 18;9:641841. doi: 10.3389/fped.2021.641841. eCollection 2021. Front Pediatr. 2021. PMID: 33681112 Free PMC article.
-
Molecular basis for chromatin binding and regulation of MLL5.Proc Natl Acad Sci U S A. 2013 Jul 9;110(28):11296-301. doi: 10.1073/pnas.1310156110. Epub 2013 Jun 24. Proc Natl Acad Sci U S A. 2013. PMID: 23798402 Free PMC article.
-
Mouse BRWD1 is critical for spermatid postmeiotic transcription and female meiotic chromosome stability.J Cell Biol. 2015 Jan 5;208(1):53-69. doi: 10.1083/jcb.201404109. Epub 2014 Dec 29. J Cell Biol. 2015. PMID: 25547156 Free PMC article.
-
MLL5 maintains spindle bipolarity by preventing aberrant cytosolic aggregation of PLK1.J Cell Biol. 2016 Mar 28;212(7):829-43. doi: 10.1083/jcb.201501021. Epub 2016 Mar 21. J Cell Biol. 2016. PMID: 27002166 Free PMC article.
-
Defining Biological and Biochemical Functions of Noncanonical SET Domain Proteins.J Mol Biol. 2024 Apr 1;436(7):168318. doi: 10.1016/j.jmb.2023.168318. Epub 2023 Oct 19. J Mol Biol. 2024. PMID: 37863247 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

