Mechanosensitive mechanisms in transcriptional regulation
- PMID: 22797927
- PMCID: PMC3434847
- DOI: 10.1242/jcs.093005
Mechanosensitive mechanisms in transcriptional regulation
Abstract
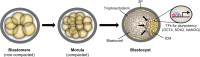
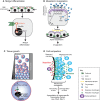
Transcriptional regulation contributes to the maintenance of pluripotency, self-renewal and differentiation in embryonic cells and in stem cells. Therefore, control of gene expression at the level of transcription is crucial for embryonic development, as well as for organogenesis, functional adaptation, and regeneration in adult tissues and organs. In the past, most work has focused on how transcriptional regulation results from the complex interplay between chemical cues, adhesion signals, transcription factors and their co-regulators during development. However, chemical signaling alone is not sufficient to explain how three-dimensional (3D) tissues and organs are constructed and maintained through the spatiotemporal control of transcriptional activities. Accumulated evidence indicates that mechanical cues, which include physical forces (e.g. tension, compression or shear stress), alterations in extracellular matrix (ECM) mechanics and changes in cell shape, are transmitted to the nucleus directly or indirectly to orchestrate transcriptional activities that are crucial for embryogenesis and organogenesis. In this Commentary, we review how the mechanical control of gene transcription contributes to the maintenance of pluripotency, determination of cell fate, pattern formation and organogenesis, as well as how it is involved in the control of cell and tissue function throughout embryogenesis and adult life. A deeper understanding of these mechanosensitive transcriptional control mechanisms should lead to new approaches to tissue engineering and regenerative medicine.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

