Proteolytic processing of dentin sialophosphoprotein (DSPP) is essential to dentinogenesis
- PMID: 22798071
- PMCID: PMC3436292
- DOI: 10.1074/jbc.M112.388587
Proteolytic processing of dentin sialophosphoprotein (DSPP) is essential to dentinogenesis
Abstract
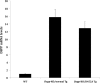
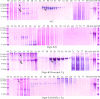
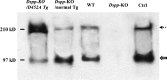
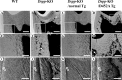
DSPP, which plays a crucial role in dentin formation, is processed into the NH(2)-terminal and COOH-terminal fragments. We believe that the proteolytic processing of DSPP is an essential activation step for its biological function in biomineralization. We tested this hypothesis by analyzing transgenic mice expressing the mutant D452A-DSPP in the Dspp-knock-out (Dspp-KO) background (referred to as "Dspp-KO/D452A-Tg" mice). We employed multipronged approaches to characterize the dentin of the Dspp-KO/D452A-Tg mice, in comparison with Dspp-KO mice and mice expressing the normal DSPP transgene in the Dspp-KO background (named Dspp-KO/normal-Tg mice). Our analyses showed that 90% of the D452A-DSPP in the dentin of Dspp-KO/D452A-Tg mice was not cleaved, indicating that D452A substitution effectively blocked the proteolytic processing of DSPP in vivo. While the expression of the normal DSPP fully rescued the dentin defects of the Dspp-KO mice, expressing the D452A-DSPP failed to do so. These results indicate that the proteolytic processing of DSPP is an activation step essential to its biological function in dentinogenesis.
Figures



References
-
- MacDougall M., Simmons D., Luan X., Nydegger J., Feng J., Gu T. T. (1997) Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J. Biol. Chem. 272, 835–842 - PubMed
-
- Butler W. T., Bhown M., Dimuzio M. T., Linde A. (1981) Nonocollagenous proteins of dentin. Isolation and partial characterization of rat dentin proteins and proteoglycans using a three-step preparative method. Coll. Relat. Res. 1, 187–199 - PubMed
-
- Veis A., Perry A. (1967) The phosphoprotein of the dentin matrix. Biochemistry 6, 2409–2416 - PubMed
-
- Xiao S., Yu C., Chou X., Yuan W., Wang Y., Bu L., Fu G., Qian M., Yang J., Shi Y., Hu L., Han B., Wang Z., Huang W., Liu J., Chen Z., Zhao G., Kong X. (2001) Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nat. Genet. 27, 201–204 - PubMed
-
- Zhang X., Zhao J., Li C., Gao S., Qiu C., Liu P., Wu G., Qiang B., Lo W. H., Shen Y. (2001) DSPP mutation in dentinogenesis imperfecta Shields type II. Nat. Genet. 27, 151–152 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

