Pharmacological stabilization of intracranial aneurysms in mice: a feasibility study
- PMID: 22798328
- PMCID: PMC3429647
- DOI: 10.1161/STROKEAHA.112.659821
Pharmacological stabilization of intracranial aneurysms in mice: a feasibility study
Abstract
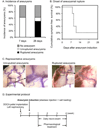
Background and purpose: An increasing number of unruptured intracranial aneurysms are being detected, partly due to the increased use of brain imaging techniques. Pharmacological stabilization of aneurysms for the prevention of aneurysmal rupture could potentially be an attractive alternative approach to clipping or coiling in patients with unruptured intracranial aneurysms. We have developed a mouse model of intracranial aneurysm that recapitulates key features of intracranial aneurysms. In this model, subarachnoid hemorrhage from aneurysmal rupture causes neurological symptoms that can be easily detected by a simple neurological examination. Using this model, we tested whether anti-inflammatory agents such as tetracycline derivatives, or a selective inhibitor of matrix metalloproteinases-2 and -9 (SB-3CT), can prevent the rupture of intracranial aneurysms.
Methods: Aneurysms were induced by a combination of induced hypertension and a single injection of elastase into the cerebrospinal fluid in mice. Treatment with minocycline, doxycycline, or SB-3CT was started 6 days after aneurysm induction. Aneurysmal rupture was detected by neurological symptoms and confirmed by the presence of intracranial aneurysms with subarachnoid hemorrhage.
Results: Minocycline and doxycycline significantly reduced rupture rates (vehicle versus doxycycline=80% versus 35%, P<0.05; vehicle versus minocycline=73% versus 24%, P<0.05) without affecting the overall incidence of aneurysms. However, SB-3CT did not affect the rupture rate (62% versus 55%, P=0.53).
Conclusions: Our data established the feasibility of using a mouse model of intracranial aneurysm to test pharmacological stabilization of aneurysms. Tetracycline derivatives could be potentially effective in preventing aneurysmal rupture.
Figures




References
-
- Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. - PubMed
-
- Berman MF, Solomon RA, Mayer SA, Johnston SC, Yung PP. Impact of hospitalrelated factors on outcome after treatment of cerebral aneurysms. Stroke. 2003;34:2200–2207. - PubMed
-
- Chyatte D, Bruno G, Desai S, Todor DR. Inflammation and intracranial aneurysms. Neurosurgery. 1999;45:1137–1146. - PubMed
-
- Shi C, Awad IA, Jafari N, Lin S, Du P, Hage ZA, et al. Genomics of human intracranial aneurysm wall. Stroke. 2009;40:1252–1261. - PubMed
-
- Kim SC, Singh M, Huang J, Prestigiacomo CJ, Winfree CJ, Solomon RA, et al. Matrix metalloproteinase-9 in cerebral aneurysms. Neurosurgery. 1997;41:642–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

