Identification of SPLUNC1's ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airways
- PMID: 22798424
- PMCID: PMC3448769
- DOI: 10.1096/fj.12-207431
Identification of SPLUNC1's ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airways
Retraction in
-
Retraction.FASEB J. 2013 May;27(5):2081. doi: 10.1096/fj.12-207431RET. FASEB J. 2013. PMID: 23632283 Free PMC article. No abstract available.
Abstract
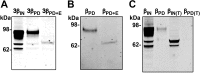
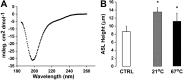
The epithelial sodium channel (ENaC) is responsible for Na+ and fluid absorption across colon, kidney, and airway epithelia. We have previously identified SPLUNC1 as an autocrine inhibitor of ENaC. We have now located the ENaC inhibitory domain of SPLUNC1 to SPLUNC1's N terminus, and a peptide corresponding to this domain, G22-A39, inhibited ENaC activity to a similar degree as full-length SPLUNC1 (∼2.5 fold). However, G22-A39 had no effect on the structurally related acid-sensing ion channels, indicating specificity for ENaC. G22-A39 preferentially bound to the β-ENaC subunit in a glycosylation-dependent manner. ENaC hyperactivity is contributory to cystic fibrosis (CF) lung disease. Addition of G22-A39 to CF human bronchial epithelial cultures (HBECs) resulted in an increase in airway surface liquid height from 4.2±0.6 to 7.9±0.6 μm, comparable to heights seen in normal HBECs, even in the presence of neutrophil elastase. Our data also indicate that the ENaC inhibitory domain of SPLUNC1 may be cleaved away from the main molecule by neutrophil elastase, which suggests that it may still be active during inflammation or neutrophilia. Furthermore, the robust inhibition of ENaC by the G22-A39 peptide suggests that this peptide may be suitable for treating CF lung disease.
Figures
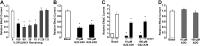
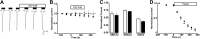


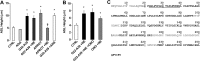
Similar articles
-
Identification of the SPLUNC1 ENaC-inhibitory domain yields novel strategies to treat sodium hyperabsorption in cystic fibrosis airway epithelial cultures.Am J Physiol Lung Cell Mol Physiol. 2013 Dec;305(12):L990-L1001. doi: 10.1152/ajplung.00103.2013. Epub 2013 Oct 11. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 24124190 Free PMC article.
-
SPLUNC1 degradation by the cystic fibrosis mucosal environment drives airway surface liquid dehydration.Eur Respir J. 2018 Oct 4;52(4):1800668. doi: 10.1183/13993003.00668-2018. Print 2018 Oct. Eur Respir J. 2018. PMID: 30190268 Free PMC article.
-
SPLUNC1 expression reduces surface levels of the epithelial sodium channel (ENaC) in Xenopus laevis oocytes.Channels (Austin). 2010 Jul-Aug;4(4):255-9. doi: 10.4161/chan.4.4.12255. Epub 2010 Jul 4. Channels (Austin). 2010. PMID: 20519934 Free PMC article.
-
Does epithelial sodium channel hyperactivity contribute to cystic fibrosis lung disease?J Physiol. 2013 Sep 15;591(18):4377-87. doi: 10.1113/jphysiol.2012.240861. Epub 2013 Jul 22. J Physiol. 2013. PMID: 23878362 Free PMC article. Review.
-
The epithelial sodium channel (ENaC) as a therapeutic target for cystic fibrosis lung disease.Expert Opin Ther Targets. 2018 Aug;22(8):687-701. doi: 10.1080/14728222.2018.1501361. Epub 2018 Jul 26. Expert Opin Ther Targets. 2018. PMID: 30028216 Review.
Cited by
-
Reduced sodium transport with nasal administration of the prostasin inhibitor camostat in subjects with cystic fibrosis.Chest. 2013 Jul;144(1):200-207. doi: 10.1378/chest.12-2431. Chest. 2013. PMID: 23412700 Free PMC article. Clinical Trial.
-
Structural Features Essential to the Antimicrobial Functions of Human SPLUNC1.Biochemistry. 2016 May 31;55(21):2979-91. doi: 10.1021/acs.biochem.6b00271. Epub 2016 May 17. Biochemistry. 2016. PMID: 27145151 Free PMC article.
-
Structural characterization of the pulmonary innate immune protein SPLUNC1 and identification of lipid ligands.FASEB J. 2014 Dec;28(12):5349-60. doi: 10.1096/fj.14-259291. Epub 2014 Sep 15. FASEB J. 2014. PMID: 25223608 Free PMC article.
-
Dual acute proinflammatory and antifibrotic pulmonary effects of short palate, lung, and nasal epithelium clone-1 after exposure to carbon nanotubes.Am J Respir Cell Mol Biol. 2013 Nov;49(5):759-67. doi: 10.1165/rcmb.2012-0435OC. Am J Respir Cell Mol Biol. 2013. PMID: 23721177 Free PMC article.
-
Inhaled Biologicals for the Treatment of Cystic Fibrosis.Recent Pat Inflamm Allergy Drug Discov. 2019;13(1):19-26. doi: 10.2174/1872213X12666181012101444. Recent Pat Inflamm Allergy Drug Discov. 2019. PMID: 30318010 Free PMC article. Review.
References
-
- Garty H., Palmer L. G. (1997) Epithelial sodium channels: function, structure, and regulation. Physiol. Rev. 77, 359–396 - PubMed
-
- Ratjen F. (2006) Restoring airway surface liquid in cystic fibrosis. N. Engl. J. Med. 354, 291–293 - PubMed
-
- Stutts M. J., Canessa C. M., Olsen J. C., Hamrick M., Cohn J. A., Rossier B. C., Boucher R. C. (1995) CFTR as a cAMP-dependent regulator of sodium channels. Science 269, 847–850 - PubMed
-
- Rowe S. M., Miller S., Sorscher E. J. (2005) Cystic fibrosis. N. Engl. J. Med. 352, 1992–2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

