Energy-dependent modulation of glucagon-like signaling in Drosophila via the AMP-activated protein kinase
- PMID: 22798489
- PMCID: PMC3454876
- DOI: 10.1534/genetics.112.143610
Energy-dependent modulation of glucagon-like signaling in Drosophila via the AMP-activated protein kinase
Abstract
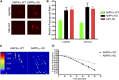
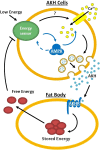
Adipokinetic hormone (AKH) is the equivalent of mammalian glucagon, as it is the primary insect hormone that causes energy mobilization. In Drosophila, current knowledge of the mechanisms regulating AKH signaling is limited. Here, we report that AMP-activated protein kinase (AMPK) is critical for normal AKH secretion during periods of metabolic challenges. Reduction of AMPK in AKH cells causes a suite of behavioral and physiological phenotypes resembling AKH cell ablations. Specifically, reduced AMPK function increases life span during starvation and delays starvation-induced hyperactivity. Neither AKH cell survival nor gene expression is significantly impacted by reduced AMPK function. AKH immunolabeling was significantly higher in animals with reduced AMPK function; this result is paralleled by genetic inhibition of synaptic release, suggesting that AMPK promotes AKH secretion. We observed reduced secretion in AKH cells bearing AMPK mutations employing a specific secretion reporter, confirming that AMPK functions in AKH secretion. Live-cell imaging of wild-type AKH neuroendocrine cells shows heightened excitability under reduced sugar levels, and this response was delayed and reduced in AMPK-deficient backgrounds. Furthermore, AMPK activation in AKH cells increases intracellular calcium levels in constant high sugar levels, suggesting that the underlying mechanism of AMPK action is modification of ionic currents. These results demonstrate that AMPK signaling is a critical feature that regulates AKH secretion, and, ultimately, metabolic homeostasis. The significance of these findings is that AMPK is important in the regulation of glucagon signaling, suggesting that the organization of metabolic networks is highly conserved and that AMPK plays a prominent role in these networks.
Figures




References
-
- Alesutan I., Föller M., Sopjani M., Dërmaku-Sopjani M., Zelenak C., et al. , 2011. Inhibition of the heterotetrameric K+ channel KCNQ1/KCNE1 by the AMP-activated protein kinase. Mol. Membr. Biol. 28: 79–89 - PubMed
-
- Andreelli F., Foretz M., Knauf C., Cani P. D., Perrin C., et al. , 2006. Liver adenosine monophosphate-activated kinase-alpha2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology 147: 2432–2441 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

