Incorporating RNA-seq data into the zebrafish Ensembl genebuild
- PMID: 22798491
- PMCID: PMC3460200
- DOI: 10.1101/gr.137901.112
Incorporating RNA-seq data into the zebrafish Ensembl genebuild
Abstract
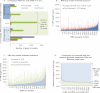
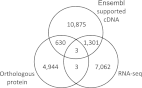
Ensembl gene annotation provides a comprehensive catalog of transcripts aligned to the reference sequence. It relies on publicly available species-specific and orthologous transcripts plus their inferred protein sequence. The accuracy of gene models is improved by increasing the species-specific component that can be cost-effectively achieved using RNA-seq. Two zebrafish gene annotations are presented in Ensembl version 62 built on the Zv9 reference sequence. Firstly, RNA-seq data from five tissues and seven developmental stages were assembled into 25,748 gene models. A 3'-end capture and sequencing protocol was developed to predict the 3' ends of transcripts, and 46.1% of the original models were subsequently refined. Secondly, a standard Ensembl genebuild, incorporating carefully filtered elements from the RNA-seq-only build, followed by a merge with the manually curated VEGA database, produced a comprehensive annotation of 26,152 genes represented by 51,569 transcripts. The RNA-seq-only and the Ensembl/VEGA genebuilds contribute contrasting elements to the final genebuild. The RNA-seq genebuild was used to adjust intron/exon boundaries of orthologous defined models, confirm their expression, and improve 3' untranslated regions. Importantly, the inferred protein alignments within the Ensembl genebuild conferred proof of model contiguity for the RNA-seq models. The zebrafish gene annotation has been enhanced by the incorporation of RNA-seq data and the pipeline will be used for other organisms. Organisms with little species-specific cDNA data will generally benefit the most.
Figures



References
-
- Cloonan N, Forrest AR, Kolle G, Gardiner BB, Faulkner GJ, Brown MK, Taylor DF, Steptoe AL, Wani S, Bethel G, et al. 2008. Stem cell transcriptome profiling via massive-scale mRNA sequencing. Nat Methods 5: 613–619 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
