The solution structure of double helical arabino nucleic acids (ANA and 2'F-ANA): effect of arabinoses in duplex-hairpin interconversion
- PMID: 22798499
- PMCID: PMC3467067
- DOI: 10.1093/nar/gks672
The solution structure of double helical arabino nucleic acids (ANA and 2'F-ANA): effect of arabinoses in duplex-hairpin interconversion
Abstract
We report here the first structure of double helical arabino nucleic acid (ANA), the C2'-stereoisomer of RNA, and the 2'-fluoro-ANA analogue (2'F-ANA). A chimeric dodecamer based on the Dickerson sequence, containing a contiguous central segment of arabino nucleotides, flanked by two 2'-deoxy-2'F-ANA wings was studied. Our data show that this chimeric oligonucleotide can adopt two different structures of comparable thermal stabilities. One structure is a monomeric hairpin in which the stem is formed by base paired 2'F-ANA nucleotides and the loop by unpaired ANA nucleotides. The second structure is a bimolecular duplex, with all the nucleotides (2'F-ANA and ANA) forming Watson-Crick base pairs. The duplex structure is canonical B-form, with all arabinoses adopting a pure C2'-endo conformation. In the ANA:ANA segment, steric interactions involving the 2'-OH substituent provoke slight changes in the glycosidic angles and, therefore, in the ANA:ANA base pair geometry. These distortions are not present in the 2'F-ANA:2'F-ANA regions of the duplex, where the -OH substituent is replaced by a smaller fluorine atom. 2'F-ANA nucleotides adopt the C2'-endo sugar pucker and fit very well into the geometry of B-form duplex, allowing for favourable 2'F···H8 interactions. This interaction shares many features of pseudo-hydrogen bonds previously observed in 2'F-ANA:RNA hybrids and in single 2'F-ANA nucleotides.
Figures
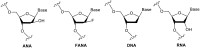
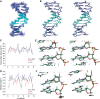

References
-
- Noronha AM, Wilds CJ, Lok CN, et al. Synthesis and biophysical properties of arabinonucleic acids (ANA): circular dichroic spectra, melting temperatures, and ribonuclease H susceptibility of ANA·RNA hybrid duplexes. Biochemistry. 2000;39:7050–7062. - PubMed
-
- Watts JK, Damha MJ. 2′F-arabinonucleic acids (2′F-ANA)-history, properties, and new frontiers. Can. J. Chem. 2008;86:641–656.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

