25-Hydroxyvitamin D response to cholecalciferol supplementation in hemodialysis
- PMID: 22798536
- PMCID: PMC3430950
- DOI: 10.2215/CJN.12761211
25-Hydroxyvitamin D response to cholecalciferol supplementation in hemodialysis
Abstract
Background and objectives: Recent understanding of extrarenal production of calcitriol has led to the exploration of native vitamin D treatment in dialysis patients. This paper reports the pharmacokinetics of 25-hydroxyvitamin D response to 10,333 IU cholecalciferol given weekly in subjects on chronic dialysis.
Design, setting, participants, & measurements: This randomized, double-blind, placebo-controlled trial of 15 weeks of oral cholecalciferol in subjects with stage 5 CKD requiring maintenance hemodialysis was conducted from November of 2007 to March of 2010. The time course of serum 25-hydroxyvitamin D was measured over the course of treatment. Additionally, blood was drawn at baseline and last visit for calcium, phosphorus, calcitriol, and parathyroid hormone levels.
Results: The median (interquartile range) baseline 25-hydroxyvitamin D level was 13.3 (11.1-16.2) ng/ml for the treatment group and 15.2 (10.7-19.9) ng/ml for the placebo group. 25-hydroxyvitamin D steady state levels rose by 23.6 (19.2-29.9) ng/ml in the treatment group, and there was no change in the placebo group. Calcitriol levels also increased significantly in the treatment group. There were no significant changes in levels of calcium, albumin, phosphorus, and parathyroid hormone in either group.
Conclusions: Cholecalciferol (10,333 IU) given weekly in patients on chronic hemodialysis produces a steady state in 25-hydroxyvitamin D of approximately 24 ng/ml.
Trial registration: ClinicalTrials.gov NCT00511225.
Figures
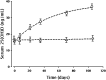
 , and the lower curve is fit to a straight line. Figure courtesy of R.P. Heaney.
, and the lower curve is fit to a straight line. Figure courtesy of R.P. Heaney.References
-
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL: Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311: 1770–1773, 2006 - PubMed
-
- Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW: The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J Bone Miner Res 13: 325–349, 1998 - PubMed
-
- National Academy of Sciences : Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Washington, DC, 2011
-
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine Society : Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96: 1911–1930, 2011 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

