Dystrophin and utrophin expression require sarcospan: loss of α7 integrin exacerbates a newly discovered muscle phenotype in sarcospan-null mice
- PMID: 22798625
- PMCID: PMC3459462
- DOI: 10.1093/hmg/dds271
Dystrophin and utrophin expression require sarcospan: loss of α7 integrin exacerbates a newly discovered muscle phenotype in sarcospan-null mice
Abstract
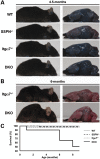
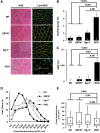
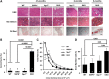
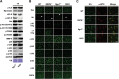
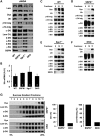
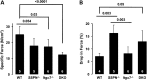
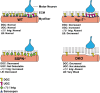
Sarcospan (SSPN) is a core component of the major adhesion complexes in skeletal muscle, the dystrophin- and utrophin (Utr)-glycoprotein complexes (DGC and UGC). We performed a rigorous analysis of SSPN-null mice and discovered that loss of SSPN decreased DGC and UGC abundance, leading to impaired laminin-binding activity and susceptibility to eccentric contraction-induced injury in skeletal muscle. We show that loss of SSPN increased levels of α7β1 integrin. To genetically test whether integrin compensates for the loss of DGC and UGC function in SSPN-nulls, we generated mice lacking both SSPN and α7 integrin (DKO, double knockout). Muscle regeneration, sarcolemma integrity and fibrosis were exacerbated in DKO mice and were remarkably similar to muscle from Duchenne muscular dystrophy (DMD) patients, suggesting that secondary loss of integrin contributes significantly to pathogenesis. Expression of the DGC and UGC, laminin binding and Akt signaling were negatively impacted in DKO muscle, resulting in severely diminished specific force properties. We demonstrate that SSPN is a necessary component of dystrophin and Utr function and that SSPN modulation of integrin signaling is required for extracellular matrix attachment and muscle force development.
Figures

References
-
- Bonnemann C.G., Finkel R.S. Sarcolemmal proteins and the spectrum of limb-girdle muscular dystrophies. Semin. Pediatr. Neurol. 2002;9:81–99. doi:10.1053/spen.2002.33795. - DOI - PubMed
-
- Carmignac V., Durbeej M. Cell-matrix interactions in muscle disease. J. Pathol. 2012;226:200–218. doi:10.1002/path.3020. - DOI - PubMed
-
- Ervasti J.M. Dystrophin, its interactions with other proteins, and implications for muscular dystrophy. Biochem. Biophys. Acta. 2007;1772:108–117. - PubMed
-
- Ibraghimov-Beskrovnaya O., Ervasti J.M., Leveille C.J., Slaughter C.A., Sernett S.W., Campbell K.P. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi:10.1038/355696a0. - DOI - PubMed
-
- Ibraghimov-Beskrovnaya O., Milatovich A., Ozcelik T., Yang B., Koepnick K., Francke U., Campbell K.P. Human dystroglycan: skeletal muscle cDNA, genomic structure, origin of tissue specific isoforms and chromosomal localization. Hum. Mol. Genet. 1993;2:1651–1657. doi:10.1093/hmg/2.10.1651. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

