Proteomic, genomic and translational approaches identify CRMP1 for a role in schizophrenia and its underlying traits
- PMID: 22798627
- PMCID: PMC3529585
- DOI: 10.1093/hmg/dds273
Proteomic, genomic and translational approaches identify CRMP1 for a role in schizophrenia and its underlying traits
Abstract
Schizophrenia is a chronic illness of heterogenous biological origin. We hypothesized that, similar to chronic progressive brain conditions, persistent functional disturbances of neurons would result in disturbed proteostasis in the brains of schizophrenia patients, leading to increased abundance of specific misfolded, insoluble proteins. Identification of such proteins would facilitate the elucidation of molecular processes underlying these devastating conditions. We therefore generated antibodies against pooled insoluble proteome of post-mortem brains from schizophrenia patients in order to identify unique, disease-specific epitopes. We successfully identified such an epitope to be present on collapsin-response mediator protein 1 (CRMP1) in biochemically purified, insoluble brain fractions. A genetic association analysis for the CRMP1 gene in a large Finnish population cohort (n = 4651) corroborated the association of physical and social anhedonia with the CRMP1 locus in a DISC1 (Disrupted-in-schizophrenia 1)-dependent manner. Physical and social anhedonia are heritable traits, present as chronic, negative symptoms of schizophrenia and severe major depression, thus constituting serious vulnerability factors for mental disease. Strikingly, lymphoblastoid cell lines derived from schizophrenia patients mirrored aberrant CRMP1 immunoreactivity by showing an increase of CRMP1 expression, suggesting its potential role as a blood-based diagnostic marker. CRMP1 is a novel candidate protein for schizophrenia traits at the intersection of the reelin and DISC1 pathways that directly and functionally interacts with DISC1. We demonstrate the impact of an interdisciplinary approach where the identification of a disease-associated epitope in post-mortem brains, powered by a genetic association study, is rapidly translated into a potential blood-based diagnostic marker.
Figures
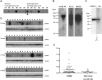
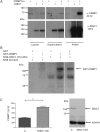
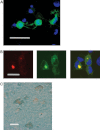
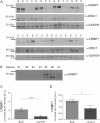
References
-
- Freimer N., Sabatti C. The human phenome project. Nat. Genet. 2003;34:15–21. - PubMed
-
- Gottesman I.I., Gould T.D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry. 2003;160:636–645. - PubMed
-
- Fanous A., Gardner C., Walsh D., Kendler K.S. Relationship between positive and negative symptoms of schizophrenia and schizotypal symptoms in nonpsychotic relatives. Arch. Gen. Psychiatry. 2001;58:669–673. - PubMed
-
- Vollema M.G., Sitskoorn M.M., Appels M.C., Kahn R.S. Does the Schizotypal Personality Questionnaire reflect the biological-genetic vulnerability to schizophrenia? Schizophr. Res. 2002;54:39–45. - PubMed
-
- Kendler K.S., McGuire M., Gruenberg A.M., O'Hare A., Spellman M., Walsh D. The Roscommon Family Study. I. Methods, diagnosis of probands, and risk of schizophrenia in relatives. Arch. Gen. Psychiatry. 1993;50:527–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 MH094268/MH/NIMH NIH HHS/United States
- MH-069853/MH/NIMH NIH HHS/United States
- MH-092443/MH/NIMH NIH HHS/United States
- R01 MH069853/MH/NIMH NIH HHS/United States
- R21 MH085226/MH/NIMH NIH HHS/United States
- MH-084018/MH/NIMH NIH HHS/United States
- MH-085226/MH/NIMH NIH HHS/United States
- MH-091460/MH/NIMH NIH HHS/United States
- MH-094268/MH/NIMH NIH HHS/United States
- RC1 MH088753/MH/NIMH NIH HHS/United States
- R01 MH092443/MH/NIMH NIH HHS/United States
- P20 MH084018/MH/NIMH NIH HHS/United States
- R01 MH091460/MH/NIMH NIH HHS/United States
- MH-088753/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

