A coreceptor-independent transgenic human TCR mediates anti-tumor and anti-self immunity in mice
- PMID: 22798675
- PMCID: PMC3674773
- DOI: 10.4049/jimmunol.1103271
A coreceptor-independent transgenic human TCR mediates anti-tumor and anti-self immunity in mice
Abstract
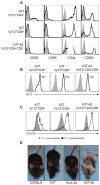
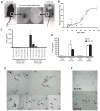
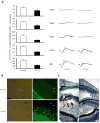
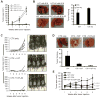
Recent advancements in T cell immunotherapy suggest that T cells engineered with high-affinity TCR can offer better tumor regression. However, whether a high-affinity TCR alone is sufficient to control tumor growth, or the T cell subset bearing the TCR is also important remains unclear. Using the human tyrosinase epitope-reactive, CD8-independent, high-affinity TCR isolated from MHC class I-restricted CD4(+) T cells obtained from tumor-infiltrating lymphocytes (TIL) of a metastatic melanoma patient, we developed a novel TCR transgenic mouse with a C57BL/6 background. This HLA-A2-restricted TCR was positively selected on both CD4(+) and CD8(+) single-positive cells. However, when the TCR transgenic mouse was developed with a HLA-A2 background, the transgenic TCR was primarily expressed by CD3(+)CD4(-)CD8(-) double-negative T cells. TIL 1383I TCR transgenic CD4(+), CD8(+), and CD4(-)CD8(-) T cells were functional and retained the ability to control tumor growth without the need for vaccination or cytokine support in vivo. Furthermore, the HLA-A2(+)/human tyrosinase TCR double-transgenic mice developed spontaneous hair depigmentation and had visual defects that progressed with age. Our data show that the expression of the high-affinity TIL 1383I TCR alone in CD3(+) T cells is sufficient to control the growth of murine and human melanoma, and the presence or absence of CD4 and CD8 coreceptors had little effect on its functional capacity.
Figures


References
-
- Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–513. - PubMed
-
- Duval L, Schmidt H, Kaltoft K, Fode K, Jensen JJ, Sorensen SM, Nishimura MI, von der Maase H. Adoptive transfer of allogeneic cytotoxic T lymphocytes equipped with a HLA-A2 restricted MART-1 T-cell receptor: a phase I trial in metastatic melanoma. Clin Cancer Res. 2006;12:1229–1236. - PubMed
-
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science (New York N Y. 2006;314:126–129. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

