Preparation and characterization of EGCase I, applicable to the comprehensive analysis of GSLs, using a rhodococcal expression system
- PMID: 22798689
- PMCID: PMC3435557
- DOI: 10.1194/jlr.D028951
Preparation and characterization of EGCase I, applicable to the comprehensive analysis of GSLs, using a rhodococcal expression system
Abstract
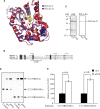
Endoglycoceramidase (EGCase) is a glycosidase capable of hydrolyzing the β -glycosidic linkage between the oligosaccharides and ceramides of glycosphingolipids (GSLs). Three molecular species of EGCase differing in specificity were found in the culture fluid of Rhodococcus equi (formerly Rhodococcus sp. M-750) and designated EGCase I, II, and III. This study describes the molecular cloning of EGCase I and characterization of the recombinant enzyme, which was highly expressed in a rhodococcal expression system using Rhodococcus erythropolis. Kinetic analysis revealed the turnover number (k(cat)) (k(cat)) of the recombinant EGCase I to be 22- and 1,200-fold higher than that of EGCase II toward GM1a and Gb3Cer, respectively, although the K(m) of both enzymes was almost the same for these substrates. Comparison of the three-dimensional structure of EGCase I (model) and EGCase II (crystal) indicated that a flexible loop hangs over the catalytic cleft of EGCase II but not EGCase I. Deletion of the loop from EGCase II increased the k(cat) of the mutant enzyme, suggesting that the loop is a critical factor affecting the turnover of substrates and products in the catalytic region. Recombinant EGCase I exhibited broad specificity and good reaction efficiency compared with EGCase II, making EGCase I well-suited to a comprehensive analysis of GSLs.
Figures



Similar articles
-
Cloning and expression of gene encoding a novel endoglycoceramidase of Rhodococcus sp. strain C9.J Biochem. 2000 Jul;128(1):145-52. doi: 10.1093/oxfordjournals.jbchem.a022725. J Biochem. 2000. PMID: 10876169
-
Structural Insights into the Broad Substrate Specificity of a Novel Endoglycoceramidase I Belonging to a New Subfamily of GH5 Glycosidases.J Biol Chem. 2017 Mar 24;292(12):4789-4800. doi: 10.1074/jbc.M116.763821. Epub 2017 Feb 8. J Biol Chem. 2017. PMID: 28179425 Free PMC article.
-
Functions and applications of glycolipid-hydrolyzing microbial glycosidases.Biosci Biotechnol Biochem. 2022 Jul 22;86(8):974-984. doi: 10.1093/bbb/zbac089. Biosci Biotechnol Biochem. 2022. PMID: 35675217 Review.
-
Activator proteins for glycosphingolipid hydrolysis by endoglycoceramidases. Elucidation of biological functions of cell-surface glycosphingolipids in situ by endoglycoceramidases made possible using these activator proteins.J Biol Chem. 1991 Apr 25;266(12):7919-26. J Biol Chem. 1991. PMID: 1850427
-
Degradation of glycolipids by endoglycoceramidase.2021 Sep 29 [updated 2022 Mar 21]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. 2021 Sep 29 [updated 2022 Mar 21]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. PMID: 37590595 Free Books & Documents. Review. No abstract available.
Cited by
-
Alteration of the Total Cellular Glycome during Late Differentiation of Chondrocytes.Int J Mol Sci. 2019 Jul 19;20(14):3546. doi: 10.3390/ijms20143546. Int J Mol Sci. 2019. PMID: 31331074 Free PMC article.
-
Vesicular Ganglioside GM1 From Breast Tumor Cells Stimulated Epithelial-to-Mesenchymal Transition of Recipient MCF-10A Cells.Front Oncol. 2022 Apr 26;12:837930. doi: 10.3389/fonc.2022.837930. eCollection 2022. Front Oncol. 2022. PMID: 35558506 Free PMC article.
-
Recent advances in cellular glycomic analyses.Biomolecules. 2013 Feb 21;3(1):198-225. doi: 10.3390/biom3010198. Biomolecules. 2013. PMID: 24970165 Free PMC article.
-
Advances in total glycomic analysis including sialylated sub-glycan isomers by SALSA method.BBA Adv. 2025 Jan 28;7:100144. doi: 10.1016/j.bbadva.2025.100144. eCollection 2025. BBA Adv. 2025. PMID: 40094062 Free PMC article.
-
Profiling Intact Glycosphingolipids with Automated Structural Annotation and Quantitation from Human Samples with Nanoflow Liquid Chromatography Mass Spectrometry.Anal Chem. 2024 Apr 16;96(15):5951-5959. doi: 10.1021/acs.analchem.4c00077. Epub 2024 Apr 2. Anal Chem. 2024. PMID: 38563595 Free PMC article.
References
-
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131: 861–872 - PubMed
-
- Wiegandt H., Bucking H. W. 1970. Carbohydrate components of extraneuronal gangliosides from bovine and human spleen, and bovine kidney. Eur. J. Biochem. 15: 287–292 - PubMed
-
- Hakomori S. I. 1966. Release of carbohydrates from sphingoglycolipid by osmium-catalyzed periodate oxidation followed by treatment with mild alkali. J. Lipid Res. 7: 789–792 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

