Characterization of Antibodies that Detect Human GFAP after Traumatic Brain Injury
- PMID: 22798722
- PMCID: PMC3394595
- DOI: 10.4137/BMI.S9873
Characterization of Antibodies that Detect Human GFAP after Traumatic Brain Injury
Abstract
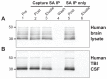
After traumatic brain injury (TBI), glial fibrillary acidic protein (GFAP) and other brain-derived proteins and their breakdown products are released into biofluids such as CSF and blood. Recently, a sandwich ELISA was constructed that measured GFAP concentrations in CSF or serum from human mild-moderate TBI patients. The goals of the present study were to characterize the same two antibodies used in this ELISA, and to determine which GFAP bands are detected by this antibody combination. Here, both antibodies recognized GFAP specifically in human brain and post-TBI CSF in a cluster of bands ranging from 50-38 kDa, that resembled bands from calpain-cleaved GFAP. By immunoprecipitation, the anti-GFAP Capture antibody recovered full length GFAP and its breakdown products from human brain lysate and post-TBI CSF. These findings demonstrate that the anti-GFAP ELISA antibodies non-preferentially detect intact GFAP and GFAP breakdown products, underscoring their utility for detecting brain injury in human patients.
Keywords: GFAP; GFAP-BDP; human; traumatic brain injury.
Figures




References
-
- Williams AJ, Hartings JA, Lu XC, Rolli ML, Tortella FC. Penetrating ballistic-like brain injury in the rat: differential time courses of hemorrhage, cell death, inflammation, and remote degeneration. J Neurotrauma. 2006 Dec;23(12):1828–46. - PubMed
-
- Wang KK. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000 Jan;23(1):20–6. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

