Chemical Biology, Molecular Mechanism and Clinical Perspective of γ-Secretase Modulators in Alzheimer's Disease
- PMID: 22798753
- PMCID: PMC3391656
- DOI: 10.2174/157015911798376352
Chemical Biology, Molecular Mechanism and Clinical Perspective of γ-Secretase Modulators in Alzheimer's Disease
Abstract
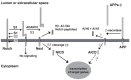
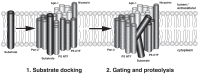
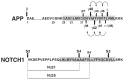
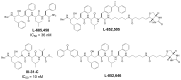
Comprehensive evidence supports that oligomerization and accumulation of amyloidogenic Aβ42 peptides in brain is crucial in the pathogenesis of both familial and sporadic forms of Alzheimer's disease. Imaging studies indicate that the buildup of Aβ begins many years before the onset of clinical symptoms, and that subsequent neurodegeneration and cognitive decline may proceed independently of Aβ. This implies the necessity for early intervention in cognitively normal individuals with therapeutic strategies that prioritize safety. The aspartyl protease γ-secretase catalyses the last step in the cellular generation of Aβ42 peptides, and is a principal target for anti-amyloidogenic intervention strategies. Due to the essential role of γ-secretase in the NOTCH signaling pathway, overt mechanism-based toxicity has been observed with the first generation of γ-secretase inhibitors, and safety of this approach has been questioned. However, two new classes of small molecules, γ-secretase modulators (GSMs) and NOTCH-sparing γ-secretase inhibitors, have revitalized γ-secretase as a drug target in AD. GSMs are small molecules that cause a product shift from Aβ42 towards shorter and less toxic Ab peptides. Importantly, GSMs spare other physiologically important substrates of the γ-secretase complex like NOTCH. Recently, GSMs with nanomolar potency and favorable in vivo properties have been described. In this review, we summarize the knowledge about the unusual proteolytic activity of γ-secretase, and the chemical biology, molecular mechanisms and clinical perspective of compounds that target the γ-secretase complex, with a particular focus on GSMs.
Keywords: Alzheimer's disease; amyloid-β peptide; gamma-secretase; gamma-secretase modulators.; neurodegeneration.
Figures
Similar articles
-
Presenilin is the molecular target of acidic γ-secretase modulators in living cells.PLoS One. 2012;7(1):e30484. doi: 10.1371/journal.pone.0030484. Epub 2012 Jan 6. PLoS One. 2012. PMID: 22238696 Free PMC article.
-
Development and mechanism of γ-secretase modulators for Alzheimer's disease.Biochemistry. 2013 May 14;52(19):3197-216. doi: 10.1021/bi400377p. Epub 2013 May 2. Biochemistry. 2013. PMID: 23614767 Free PMC article. Review.
-
Second generation γ-secretase modulators exhibit different modulation of Notch β and Aβ production.J Biol Chem. 2012 Sep 21;287(39):32640-50. doi: 10.1074/jbc.M112.376541. Epub 2012 Jul 31. J Biol Chem. 2012. PMID: 22851182 Free PMC article.
-
Characterization of FRM-36143 as a new γ-secretase modulator for the potential treatment of familial Alzheimer's disease.Alzheimers Res Ther. 2016 Aug 30;8(1):34. doi: 10.1186/s13195-016-0199-5. Alzheimers Res Ther. 2016. PMID: 27572246 Free PMC article.
-
Recent developments of small molecule γ-secretase modulators for Alzheimer's disease.RSC Med Chem. 2020 Aug 27;11(9):1003-1022. doi: 10.1039/d0md00196a. eCollection 2020 Sep 1. RSC Med Chem. 2020. PMID: 33479693 Free PMC article. Review.
Cited by
-
Modulation of Aβ42 in vivo by γ-secretase modulator in primates and humans.Alzheimers Res Ther. 2015 Aug 5;7(1):55. doi: 10.1186/s13195-015-0137-y. eCollection 2015. Alzheimers Res Ther. 2015. PMID: 26244059 Free PMC article.
-
Novel Anti-Alzheimer's Therapeutic Molecules Targeting Amyloid Precursor Protein Processing.Oxid Med Cell Longev. 2020 Apr 29;2020:7039138. doi: 10.1155/2020/7039138. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32411333 Free PMC article. Review.
-
Development of nanoparticles incorporating a novel liposomal membrane destabilization peptide for efficient release of cargos into cancer cells.PLoS One. 2014 Oct 24;9(10):e111181. doi: 10.1371/journal.pone.0111181. eCollection 2014. PLoS One. 2014. PMID: 25343714 Free PMC article.
-
R-flurbiprofen improves tau, but not Aß pathology in a triple transgenic model of Alzheimer's disease.Brain Res. 2013 Dec 6;1541:115-27. doi: 10.1016/j.brainres.2013.10.025. Epub 2013 Oct 22. Brain Res. 2013. PMID: 24161403 Free PMC article.
-
Modulation of gamma-secretase for the treatment of Alzheimer's disease.Int J Alzheimers Dis. 2012;2012:210756. doi: 10.1155/2012/210756. Epub 2012 Dec 19. Int J Alzheimers Dis. 2012. PMID: 23320246 Free PMC article.
References
-
- Wancata J, Musalek M, Alexandrowicz R, Krautgartner M. Number of dementia sufferers in Europe between the years 2000 and 2050. Eur. Psychiatry. 2003;18(6):306–313. - PubMed
-
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81(2):741–766. - PubMed
-
- Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J. Neurochem. 2009;110:1129–1134. - PubMed
-
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimers disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimers disease. Nat. Med. 1996;2(8):864–869. - PubMed
LinkOut - more resources
Full Text Sources
