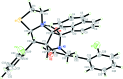
11-[(E)-2-Fluoro-benzyl-idene]-8-(2-fluoro-phen-yl)-14-hy-droxy-6-thia-3,13-diaza-hepta-cyclo-[13.7.1.1(9,13).0(2,9).0(2,14).0(3,7).0(19,23)]tetra-cosa-1(22),15(23),16,18,20-pentaen-10-one
- PMID: 22798773
- PMCID: PMC3393908
- DOI: 10.1107/S1600536812025512
11-[(E)-2-Fluoro-benzyl-idene]-8-(2-fluoro-phen-yl)-14-hy-droxy-6-thia-3,13-diaza-hepta-cyclo-[13.7.1.1(9,13).0(2,9).0(2,14).0(3,7).0(19,23)]tetra-cosa-1(22),15(23),16,18,20-pentaen-10-one
Abstract
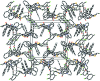
In the title compound, C(34)H(26)F(2)N(2)O(2)S, an intra-molecular O-H⋯N hydrogen bond forms an S(5) ring motif. The piperidine ring adopts a chair conformation. The thia-zolidine ring and one of the pyrrolidine rings adopt envolope conformations with methyl-ene C atoms at the flap, whereas the other pyrrolidine ring adopts a half-chair conformation. The fluoro-substituted benzene rings form dihedral angles of 32.25 (10) and 38.27 (10)°, respectively, with the mean plane of the dihydro-acenaphthyl-ene ring system [maximum deviation = 0.043 (2) Å]. The dihedral angle between the fluoro-substituted benzene rings is 64.13 (14)°. In the crystal, mol-ecules are linked by weak C-H⋯O, C-H⋯F and C-H⋯S hydrogen bonds into a three-dimensional network.
Figures
References
-
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
-
- Bruker (2009). SADABS, APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst. 19, 105–107.
-
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
-
- Kirsch, G., Hesse, S. & Comel, A. (2004). Curr. Org. Chem. 1, 47–63.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous