2-Amino-4-(4-meth-oxy-phen-yl)-7,7-di-methyl-5-oxo-5,6,7,8-tetra-hydro-4H-chromene-3-carbonitrile propan-2-one monosolvate
- PMID: 22798868
- PMCID: PMC3394003
- DOI: 10.1107/S160053681202781X
2-Amino-4-(4-meth-oxy-phen-yl)-7,7-di-methyl-5-oxo-5,6,7,8-tetra-hydro-4H-chromene-3-carbonitrile propan-2-one monosolvate
Abstract
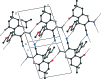
In the crystal structure of the title compound, C(19)H(20)N(2)O(3)·C(3)H(6)O, mol-ecules are linked into inversion dimers with an R(2) (2)(12) motif by pairs of N-H⋯N hydrogen bonds. These dimers are further connected into chains running along the a axis by N-H⋯O hydrogen bonds. C-H⋯N and C-H⋯π inter-actions also feature in the packing. The cyclohexene ring adopts nearly an envelope conformation [puckering parameters are Q(T) = 0.456 (2) Å, θ = 54.6 (3)° and ϕ = 225.2 (3)°].
Figures

Similar articles
-
2-Amino-4-(4-chloro-phen-yl)-7,7-dimethyl-5-oxo-5,6,7,8-tetra-hydro-4H-chromene-3-carbonitrile propan-2-one monosolvate.Acta Crystallogr Sect E Struct Rep Online. 2012 Jun 1;68(Pt 6):o1965-6. doi: 10.1107/S1600536812024142. Epub 2012 May 31. Acta Crystallogr Sect E Struct Rep Online. 2012. PMID: 22719712 Free PMC article.
-
2-Amino-4-(4-methyl-phen-yl)-5-oxo-5,6,7,8-tetra-hydro-4H-chromene-3-carbonitrile.Acta Crystallogr Sect E Struct Rep Online. 2012 Aug 1;68(Pt 8):o2315-6. doi: 10.1107/S1600536812029480. Epub 2012 Jul 4. Acta Crystallogr Sect E Struct Rep Online. 2012. PMID: 22904790 Free PMC article.
-
2-Amino-4-(4-meth-oxy-phen-yl)-5-oxo-5,6,7,8-tetra-hydro-4H-chromene-3-carbonitrile 1,4-dioxane hemisolvate.Acta Crystallogr Sect E Struct Rep Online. 2012 Jul 1;68(Pt 7):o2178-9. doi: 10.1107/S1600536812027729. Epub 2012 Jun 23. Acta Crystallogr Sect E Struct Rep Online. 2012. PMID: 22798847 Free PMC article.
-
Crystal structure of ethyl 6-chloro-methyl-2-oxo-4-(2,3,4-tri-meth-oxy-phen-yl)-1,2,3,4-tetra-hydro-pyrimidine-5-carboxyl-ate.Acta Crystallogr E Crystallogr Commun. 2015 Jun 20;71(Pt 7):821-3. doi: 10.1107/S2056989015011688. eCollection 2015 Jul 1. Acta Crystallogr E Crystallogr Commun. 2015. PMID: 26279876 Free PMC article.
-
2-Amino-4-(2-chloro-phen-yl)-5-oxo-5,6,7,8-tetra-hydro-4H-chromene-3-carbonitrile ethanol monosolvate.Acta Crystallogr Sect E Struct Rep Online. 2011 Nov;67(Pt 11):o3099. doi: 10.1107/S1600536811043650. Epub 2011 Oct 29. Acta Crystallogr Sect E Struct Rep Online. 2011. PMID: 22220103 Free PMC article.
References
-
- Abd-El-Aziz, A. S., El-Agrody, A. M., Bedair, A. H., Corkery, T. C. & Ata, A. (2004). Heterocycles, 63, 1793–1812.
-
- Abd-El-Aziz, A. S., Mohamed, H. M., Mohammed, S., Zahid, S., Ata, A., Bedair, A. H., El- Agrody, A. M. & Harvey, P. D. (2007). J. Heterocycl. Chem. 44, 1287–1301.
-
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
-
- Brühlmann, C., Ooms, F., Carrupt, P.-A., Testa, B., Catto, M., Leonetti, F., Altomare, C. & Carotti, A. (2001). J. Med. Chem. 44, 3195–3198. - PubMed
-
- Bruker (2005). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
