2-(4-Bromo-phen-yl)-N-[3-(1H-imidazol-1-yl)prop-yl]quinazolin-4-amine
- PMID: 22798902
- PMCID: PMC3394037
- DOI: 10.1107/S1600536812028115
2-(4-Bromo-phen-yl)-N-[3-(1H-imidazol-1-yl)prop-yl]quinazolin-4-amine
Abstract
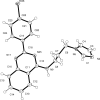
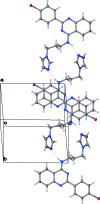
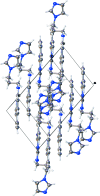
In the title compound, C(20)H(18)BrN(5), the bromo-phenyl-substituted quinazoline unit is essentially planar [maximum deviation = 0.098 (3) Å] and makes a dihedral angle of 56.04 (14)° with the imidazole ring. In the crystal, mol-ecules are associated by pairs of N-H⋯N hydrogen bonds to form inversion dimers. All the quinazoline planar systems are oriented almost perpendicular to the [110] direction, making π-π inter-actions possible between adjacent dimers [centroid-centroid distances = 3.7674 (16) and 3.7612 (17) Å]. There are also a number of C-H⋯π inter-actions present. The crystal is a nonmerohedral twin, with a minor twin fraction of 0.47.
Figures
References
-
- Agilent (2010). CrysAlis PRO Agilent Technologies, Yarnton, Oxfordshire, England.
-
- Aguilar, L., Gimenez, M. J., García-Rey, C. & Martin, J. E. (2002). Antimicrob. Chemother. 50 (Suppl. C), 93–100. - PubMed
-
- Asproni, B., Murineddu, G., Pau, A., Pinna, G. A., Langgård, M., Christoffersen, C. T., Nielsen, J. & Kehler, J. (2011). Bioorg. Med. Chem. 19, 642–649. - PubMed
-
- Connolly, D. J., Cusack, D. O., Sullivan, T. P. & Guiry, P. J. (2005). Tetrahedron, 61, 10153–10202.
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
