Physiological heterogeneities in microbial populations and implications for physical stress tolerance
- PMID: 22799461
- PMCID: PMC3443036
- DOI: 10.1186/1475-2859-11-94
Physiological heterogeneities in microbial populations and implications for physical stress tolerance
Abstract
Background: Traditionally average values of the whole population are considered when analysing microbial cell cultivations. However, a typical microbial population in a bioreactor is heterogeneous in most phenotypes measurable at a single-cell level. There are indications that such heterogeneity may be unfavourable on the one hand (reduces yields and productivities), but also beneficial on the other hand (facilitates quick adaptation to new conditions--i.e. increases the robustness of the fermentation process). Understanding and control of microbial population heterogeneity is thus of major importance for improving microbial cell factory processes.
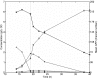
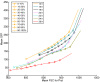
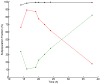
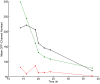
Results: In this work, a dual reporter system was developed and applied to map growth and cell fitness heterogeneities within budding yeast populations during aerobic cultivation in well-mixed bioreactors. The reporter strain, which was based on the expression of green fluorescent protein (GFP) under the control of the ribosomal protein RPL22a promoter, made it possible to distinguish cell growth phases by the level of fluorescence intensity. Furthermore, by exploiting the strong correlation of intracellular GFP level and cell membrane integrity it was possible to distinguish subpopulations with high and low cell membrane robustness and hence ability to withstand freeze-thaw stress. A strong inverse correlation between growth and cell membrane robustness was observed, which further supports the hypothesis that cellular resources are limited and need to be distributed as a trade-off between two functions: growth and robustness. In addition, the trade-off was shown to vary within the population, and the occurrence of two distinct subpopulations shifting between these two antagonistic modes of cell operation could be distinguished.
Conclusions: The reporter strain enabled mapping of population heterogeneities in growth and cell membrane robustness towards freeze-thaw stress at different phases of cell cultivation. The described reporter system is a valuable tool for understanding the effect of environmental conditions on population heterogeneity of microbial cells and thereby to understand cell responses during industrial process-like conditions. It may be applied to identify more robust subpopulations, and for developing novel strategies for strain improvement and process design for more effective bioprocessing.
Figures


References
-
- Lencastre Fernandes R, Nierychlo M, Lundin L, Pedersen AE, Puentes Tellez PE, Dutta A, Carlquist M, Bolic A, Schapper D, Brunetti AC, Helmark S, Heins AL, Jensen AD, Nopens I, Rottwitt K, Szita N, Van Elsas JD, Nielsen PH, Martinussen J, Sørensen SJ, Lantz AE, Gernaey KV. Experimental methods and modeling techniques for description of cell population heterogeneity. Biotechnol Adv. 2011;29:575–599. doi: 10.1016/j.biotechadv.2011.03.007. - DOI - PubMed
-
- Enfors SO, Jahic M, Rozkov A, Xu B, Hecker M, Jurgen B, Kruger E, Schweder T, Hamer G, O'Beirne D, Noisommit-Rizzi N, Reuss M, Boone L, Hewitt C, McFarlane C, Nienow A, Kovacs T, Trägårdh C, Fuchs L, Revstedt J, Friberg PC, Hjertager B, Blomsten G, Skogman H, Hjort S, Hoeks F, Lin HY, Neubauer P, Van der Lans R, Luyben K. et al.Physiological responses to mixing in large scale bioreactors. J Biotechnol. 2001;85:175–185. doi: 10.1016/S0168-1656(00)00365-5. - DOI - PubMed
-
- Hewitt CJ, Nebe-Von Caron G, Axelsson B, McFarlane CM, Nienow AW. Studies related to the scale-up of high-cell-density E. coli fed-batch fermentations using multiparameter flow cytometry: effect of a changing microenvironment with respect to glucose and dissolved oxygen concentration. Biotechnol Bioeng. 2000;70:381–390. doi: 10.1002/1097-0290(20001120)70:4<381::AID-BIT3>3.0.CO;2-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

