Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy
- PMID: 22800401
- PMCID: PMC3935509
- DOI: 10.1016/j.jaci.2012.05.039
Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy
Abstract
Background: Monoclonal antibodies directed at IgE demonstrate clinical efficacy in subjects with peanut allergy, but previous studies have not addressed the kinetics of the clinical response or the role of mast cells and basophils in the food-induced allergic response.
Objective: We sought to determine the kinetics of the clinical response to omalizumab and whether clinical improvement is associated with either mast cell or basophil suppression.
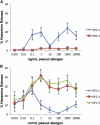
Methods: Subjects with peanut allergy were treated with omalizumab for 6 months and assessed for clinical and cellular responses. At baseline, subjects had a double-blind, placebo-controlled oral food challenge (OFC), skin prick test titration (SPTT), and basophil histamine release (BHR) to peanut. BHR was repeated at week 2 and then weekly until it decreased to less than 20% of baseline values. The OFCs and SPTTs were repeated after the BHR reduction (or at week 8 if BHR did not decrease) and again at 6 months.
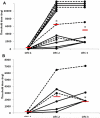
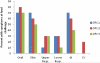
Results: Fourteen subjects enrolled in the study. At the second food challenge, there was a significant increase in the threshold dose of peanut inducing allergic symptoms (80 to 6500 mg, P < .01). Peanut-induced BHR was either completely suppressed (n = 5) or 10-fold more allergen was required to induce maximal BHR (n = 9), and SPTT responses were not significantly changed from baseline. After 6 months of omalizumab, further changes in the OFC threshold dose or BHR were not observed, but a significant suppression in SPTTs was identified.
Conclusions: The clinical response to omalizumab occurs early in treatment when the basophil, but not the mast cell, is suppressed, supporting a role for the basophil in acute food reactions.
Copyright © 2012 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures


Similar articles
-
Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge.J Allergy Clin Immunol. 2010 Apr;125(4):889-895.e7. doi: 10.1016/j.jaci.2009.09.012. Epub 2009 Dec 4. J Allergy Clin Immunol. 2010. PMID: 19962744 Free PMC article. Clinical Trial.
-
Suppression of the basophil response to allergen during treatment with omalizumab is dependent on 2 competing factors.J Allergy Clin Immunol. 2012 Nov;130(5):1130-1135.e5. doi: 10.1016/j.jaci.2012.05.038. Epub 2012 Jul 15. J Allergy Clin Immunol. 2012. PMID: 22800400 Free PMC article.
-
A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients.J Allergy Clin Immunol. 2013 Dec;132(6):1368-74. doi: 10.1016/j.jaci.2013.09.046. Epub 2013 Oct 28. J Allergy Clin Immunol. 2013. PMID: 24176117 Free PMC article. Clinical Trial.
-
Omalizumab may not inhibit mast cell and basophil activation in vitro.J Eur Acad Dermatol Venereol. 2015 Sep;29(9):1832-6. doi: 10.1111/jdv.12693. Epub 2014 Sep 26. J Eur Acad Dermatol Venereol. 2015. PMID: 25257818 Review.
-
IgE antibody-specific activity in human allergic disease.Immunol Res. 2010 Jul;47(1-3):273-84. doi: 10.1007/s12026-009-8160-3. Immunol Res. 2010. PMID: 20066506 Review.
Cited by
-
Update of the S2k guideline on the management of IgE-mediated food allergies.Allergol Select. 2021 Jul 8;5:195-243. doi: 10.5414/ALX02257E. eCollection 2021. Allergol Select. 2021. PMID: 34263109 Free PMC article.
-
Combining Anti-IgE Monoclonal Antibodies and Oral Immunotherapy for the Treatment of Food Allergy.Clin Rev Allergy Immunol. 2022 Feb;62(1):216-231. doi: 10.1007/s12016-021-08902-0. Epub 2021 Sep 22. Clin Rev Allergy Immunol. 2022. PMID: 34550555 Review.
-
Targeting the FcεRI Pathway as a Potential Strategy to Prevent Food-Induced Anaphylaxis.Front Immunol. 2020 Dec 17;11:614402. doi: 10.3389/fimmu.2020.614402. eCollection 2020. Front Immunol. 2020. PMID: 33391286 Free PMC article. Review.
-
The Role of Biologics in the Treatment of Food Allergy.J Allergy Clin Immunol Pract. 2024 Mar;12(3):562-568. doi: 10.1016/j.jaip.2023.11.032. Epub 2023 Nov 25. J Allergy Clin Immunol Pract. 2024. PMID: 38013157 Free PMC article.
-
The efficacy of omalizumab treatment in chronic spontaneous urticaria is associated with basophil phenotypes.J Allergy Clin Immunol. 2021 Jun;147(6):2271-2280.e8. doi: 10.1016/j.jaci.2021.02.038. Epub 2021 Mar 10. J Allergy Clin Immunol. 2021. PMID: 33713769 Free PMC article.
References
-
- Beck LA, Marcotte GV, MacGlashan JD, Togias A, Saini S. Omalizumab-induced reductions in mast cell FcεRI expression and function. J Allergy Clin Immunol. 2004;114:527–30. - PubMed
-
- Leung DY, Sampson HA, Yunginger JW, Burks AW, Jr, Schneider LC, Wortel CH, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986–93. - PubMed
-
- Sampson HA, Leung DY, Burks AW, Lack G, Bahna SL, Jones SM, et al. A phase II, randomized, double blind, parallel group, placebo controlled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol. 2011;127:1309–10. - PubMed
-
- Vonakis BM, Vasagar K, Gibbons JSP, Gober L, Sterba PM, Chang H, et al. Basophil FcεRI histamine release parallels expression of Src-homology 2-containing inositol phosphatases in chronic idiopathic urticaria. JAllergy Clin Immunol. 2007;119:441–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

