Enhanced drug delivery capabilities from stents coated with absorbable polymer and crystalline drug
- PMID: 22800575
- PMCID: PMC4637970
- DOI: 10.1016/j.jconrel.2012.07.004
Enhanced drug delivery capabilities from stents coated with absorbable polymer and crystalline drug
Abstract
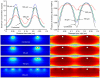
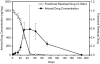
Current drug eluting stent (DES) technology is not optimized with regard to the pharmacokinetics of drug delivery. A novel, absorbable-coating sirolimus-eluting stent (AC-SES) was evaluated for its capacity to deliver drug more evenly within the intimal area rather than concentrating drug around the stent struts and for its ability to match coating erosion with drug release. The coating consisted of absorbable poly-lactide-co-glycolic acid (PLGA) and crystalline sirolimus deposited by a dry-powder electrostatic process. The AC-SES demonstrated enhanced drug stability under simulated use conditions and consistent drug delivery balanced with coating erosion in a porcine coronary implant model. The initial drug burst was eliminated and drug release was sustained after implantation. The coating was absorbed within 90 days. Following implantation into porcine coronary arteries the AC-SES coating is distributed in the surrounding intimal tissue over the course of several weeks. Computational modeling of drug delivery characteristics demonstrates how distributed coating optimizes the load of drug immediately around each stent strut and extends drug delivery between stent struts. The result was a highly efficient arterial uptake of drug with superior performance to a clinical bare metal stent (BMS). Neointimal thickness (0.17±0.07 mm vs. 0.28±0.11 mm) and area percent stenosis (22±9% vs. 35±12%) were significantly reduced (p<0.05) by the AC-SES compared to the BMS 30 days after stent implantation in an overlap configuration in porcine coronary arteries. Inflammation was significantly reduced in the AC-SES compared to the BMS at both 30 and 90 days after implantation. Biocompatible, rapidly absorbable stent coatings enable the matching of drug release with coating erosion and provide for the controlled migration of coating material into tissue to reduce vicissitudes in drug tissue levels, optimizing efficacy and reducing potential toxicity.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures





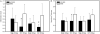

References
-
- Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schuhlen H, Neumann F-J, Fleckenstein M, Pfafferott C, Seyfarth M, Schomig A. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103:2816–2821. - PubMed
-
- Pache J, Kastrati A, Mehilli J, Schuhlen H, Dotzer F, Hausleiter J, Fleckenstein M, Neumann FJ, Sattelberger U, Schmitt C, Muller M, Dirschinger J, Schomig A. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J. Am. Coll. Cardiol. 2003;41(8):1283–1288. - PubMed
-
- Holmes DR., Jr. State of the art in coronary intervention. Am. J. Cardiol. 2003;91(3A):50A–53A. - PubMed
-
- Byrne RA, Joner M, Kastrati A. Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol. 2009;57(5):567–584. - PubMed
-
- Nakazawa G, Finn AV, Kolodgie FD, Virmani R. A review of current devices and a look at new technology: drug-eluting stents. Expert Rev. Med. Devices. 2009;6(1):33–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

