Tumor necrosis factor-alpha induces renal cyclooxygenase-2 expression in response to hypercalcemia
- PMID: 22800939
- PMCID: PMC3429635
- DOI: 10.1016/j.prostaglandins.2012.07.001
Tumor necrosis factor-alpha induces renal cyclooxygenase-2 expression in response to hypercalcemia
Abstract
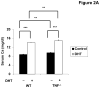
The effect of tumor necrosis factor-alpha (TNF) on cyclooxygenase-2 (COX-2) expression in the renal outer medulla (OM) was determined in a model of dihydrotachysterol (DHT)-induced hypercalcemia. Increases in serum calcium and water intake were observed during ingestion of a DHT-containing diet in both wild type (WT) and TNF deficient mice (TNF(-/-)). Polyuria and a decrease in body weight were observed in response to DHT treatment in WT and TNF(-/-) mice. A transient elevation in urinary TNF was observed in WT mice treated with DHT. Moreover, increased urinary levels of prostaglandin E(2) (PGE(2)) and a corresponding increase in COX-2 expression in the OM were observed in WT mice fed DHT. Increased COX-2 expression was not observed in TNF(-/-) mice fed DHT, and the characteristics of PGE(2) synthesis were distinct from those in WT mice. This study demonstrates that COX-2 expression in the OM, secondary to hypercalemia, is TNF-dependent.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures









References
-
- Wang D, An SJ, Wang WH, McGiff JC, Ferreri NR. CaR-mediated COX-2 expression in primary cultured mTAL cells. Am J Physiol Renal Physiol. 2001;281(4):F658–64. - PubMed
-
- Wang D, Pedraza PL, Abdullah HI, McGiff JC, Ferreri NR. Calcium-sensing receptor-mediated TNF production in medullary thick ascending limb cells. Am J Physiol Renal Physiol. 2002;283(5):F963–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

