Kinase suppressor of Ras 2 (KSR2) regulates tumor cell transformation via AMPK
- PMID: 22801368
- PMCID: PMC3430199
- DOI: 10.1128/MCB.06754-11
Kinase suppressor of Ras 2 (KSR2) regulates tumor cell transformation via AMPK
Abstract
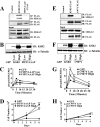
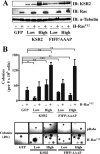
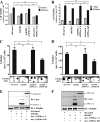
Kinase suppressor of Ras 1 (KSR1) and KSR2 are scaffolds that promote extracellular signal-regulated kinase (ERK) signaling but have dramatically different physiological functions. KSR2(-/-) mice show marked deficits in energy expenditure that cause obesity. In contrast, KSR1 disruption has inconsequential effects on development but dramatically suppresses tumor formation by activated Ras. We examined the role of KSR2 in the generation and maintenance of the transformed phenotype in KSR1(-/-) mouse embryo fibroblasts (MEFs) expressing activated Ras(V12) and in tumor cell lines MIN6 and NG108-15. KSR2 rescued ERK activation and accelerated proliferation in KSR1(-/-) MEFs. KSR2 expression alone induced anchorage-independent growth and synergized with the transforming effects of Ras(V12). Similarly, RNA interference (RNAi) of KSR2 in MIN6 and NG108-15 cells inhibited proliferation and colony formation, with concomitant defects in AMP-activated protein kinase (AMPK) signaling, nutrient metabolism, and metabolic capacity. While constitutive activation of AMPK was sufficient to complement the loss of KSR2 in metabolic signaling and anchorage-independent growth, KSR2 RNAi, MEK inhibition, and expression of a KSR2 mutant unable to interact with ERK demonstrated that mitogen-activated protein (MAP) kinase signaling is dispensable for the transformed phenotype of these cells. These data show that KSR2 is essential to tumor cell energy homeostasis and critical to the integration of mitogenic and metabolic signaling pathways.
Figures






Similar articles
-
Kinase suppressor of ras 1 (KSR1) regulates PGC1α and estrogen-related receptor α to promote oncogenic Ras-dependent anchorage-independent growth.Mol Cell Biol. 2011 Jun;31(12):2453-61. doi: 10.1128/MCB.05255-11. Epub 2011 Apr 25. Mol Cell Biol. 2011. PMID: 21518958 Free PMC article.
-
KSR2 is an essential regulator of AMP kinase, energy expenditure, and insulin sensitivity.Cell Metab. 2009 Nov;10(5):366-78. doi: 10.1016/j.cmet.2009.09.010. Cell Metab. 2009. PMID: 19883615 Free PMC article.
-
KSR2 is a calcineurin substrate that promotes ERK cascade activation in response to calcium signals.Mol Cell. 2009 Jun 26;34(6):652-62. doi: 10.1016/j.molcel.2009.06.001. Mol Cell. 2009. PMID: 19560418 Free PMC article.
-
Coordinating ERK signaling via the molecular scaffold Kinase Suppressor of Ras.F1000Res. 2017 Aug 31;6:1621. doi: 10.12688/f1000research.11895.1. eCollection 2017. F1000Res. 2017. PMID: 29026529 Free PMC article. Review.
-
KSR as a therapeutic target for Ras-dependent cancers.Expert Opin Ther Targets. 2017 May;21(5):499-509. doi: 10.1080/14728222.2017.1311325. Epub 2017 Apr 7. Expert Opin Ther Targets. 2017. PMID: 28333549 Free PMC article. Review.
Cited by
-
Two distinct classes of thymic tumors in patients with MEN1 show LOH at the MEN1 locus.Endocr Relat Cancer. 2021 Oct 4;28(11):L15-L19. doi: 10.1530/ERC-21-0226. Endocr Relat Cancer. 2021. PMID: 34515662 Free PMC article.
-
Caveolin-1 is required for kinase suppressor of Ras 1 (KSR1)-mediated extracellular signal-regulated kinase 1/2 activation, H-RasV12-induced senescence, and transformation.Mol Cell Biol. 2014 Sep 15;34(18):3461-72. doi: 10.1128/MCB.01633-13. Epub 2014 Jul 7. Mol Cell Biol. 2014. PMID: 25002533 Free PMC article.
-
A Gene Expression High-Throughput Screen (GE-HTS) for Coordinated Detection of Functionally Similar Effectors in Cancer.Cancers (Basel). 2020 Oct 27;12(11):3143. doi: 10.3390/cancers12113143. Cancers (Basel). 2020. PMID: 33120942 Free PMC article. Review.
-
Metabolic underpinnings of cancer-related fatigue.Am J Physiol Endocrinol Metab. 2024 Mar 1;326(3):E290-E307. doi: 10.1152/ajpendo.00378.2023. Epub 2024 Jan 31. Am J Physiol Endocrinol Metab. 2024. PMID: 38294698 Free PMC article. Review.
-
KSR1 Mediates Small Cell Lung Carcinoma Tumor Initiation and Cisplatin Resistance.Mol Cancer Res. 2025 Jun 3;23(6):553-566. doi: 10.1158/1541-7786.MCR-24-0652. Mol Cancer Res. 2025. PMID: 39927878
References
-
- Buzzai M, et al. 2005. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene 24:4165–4173 - PubMed
-
- Corton JM, Gillespie JG, Hawley SA, Hardie DG. 1995. 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 229:558–565 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
