Correlation between NS5A dimerization and hepatitis C virus replication
- PMID: 22801423
- PMCID: PMC3436329
- DOI: 10.1074/jbc.M112.376822
Correlation between NS5A dimerization and hepatitis C virus replication
Abstract
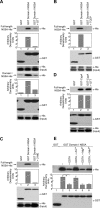
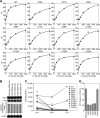
Hepatitis C virus (HCV) is the main agent of acute and chronic liver diseases leading to cirrhosis and hepatocellular carcinoma. The current standard therapy has limited efficacy and serious side effects. Thus, the development of alternate therapies is of tremendous importance. HCV NS5A (nonstructural 5A protein) is a pleiotropic protein with key roles in HCV replication and cellular signaling pathways. Here we demonstrate that NS5A dimerization occurs through Domain I (amino acids 1-240). This interaction is not mediated by nucleic acids because benzonase, RNase, and DNase treatments do not prevent NS5A-NS5A interactions. Importantly, DTT abrogates NS5A-NS5A interactions but does not affect NS5A-cyclophilin A interactions. Other reducing agents such as tris(2-carboxyethyl)phosphine and 2-mercaptoethanol also abrogate NS5A-NS5A interactions, implying that disulfide bridges may play a role in this interaction. Cyclophilin inhibitors, cyclosporine A, and alisporivir and NS5A inhibitor BMS-790052 do not block NS5A dimerization, suggesting that their antiviral effects do not involve the disruption of NS5A-NS5A interactions. Four cysteines, Cys-39, Cys-57, Cys-59, and Cys-80, are critical for dimerization. Interestingly, the four cysteines have been proposed to form a zinc-binding motif. Supporting this notion, NS5A dimerization is greatly facilitated by Zn(2+) but not by Mg(2+) or Mn(2+). Importantly, the four cysteines are vital not only for viral replication but also critical for NS5A binding to RNA, revealing a correlation between NS5A dimerization, RNA binding, and HCV replication. Altogether our data suggest that NS5A-NS5A dimerization and/or multimerization could represent a novel target for the development of HCV therapies.
Figures




Similar articles
-
Daclatasvir-like inhibitors of NS5A block early biogenesis of hepatitis C virus-induced membranous replication factories, independent of RNA replication.Gastroenterology. 2014 Nov;147(5):1094-105.e25. doi: 10.1053/j.gastro.2014.07.019. Epub 2014 Jul 18. Gastroenterology. 2014. PMID: 25046163
-
Resensitizing daclatasvir-resistant hepatitis C variants by allosteric modulation of NS5A.Nature. 2015 Nov 12;527(7577):245-8. doi: 10.1038/nature15711. Epub 2015 Nov 4. Nature. 2015. PMID: 26536115
-
ISGylation of Hepatitis C Virus NS5A Protein Promotes Viral RNA Replication via Recruitment of Cyclophilin A.J Virol. 2020 Sep 29;94(20):e00532-20. doi: 10.1128/JVI.00532-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32727878 Free PMC article.
-
Discovery of hepatitis C virus NS5A inhibitors as a new class of anti-HCV therapy.Arch Pharm Res. 2011 Sep;34(9):1403-7. doi: 10.1007/s12272-011-0921-6. Arch Pharm Res. 2011. PMID: 21975800 Review.
-
Phosphorylation of hepatitis C virus NS5A nonstructural protein: a new paradigm for phosphorylation-dependent viral RNA replication?Virology. 2007 Jul 20;364(1):1-9. doi: 10.1016/j.virol.2007.01.042. Epub 2007 Apr 2. Virology. 2007. PMID: 17400273 Review.
Cited by
-
Daclatasvir inhibits hepatitis C virus NS5A motility and hyper-accumulation of phosphoinositides.Virology. 2015 Feb;476:168-179. doi: 10.1016/j.virol.2014.12.018. Epub 2014 Dec 26. Virology. 2015. PMID: 25546252 Free PMC article.
-
Serine 229 Balances the Hepatitis C Virus Nonstructural Protein NS5A between Hypo- and Hyperphosphorylated States.J Virol. 2019 Nov 13;93(23):e01028-19. doi: 10.1128/JVI.01028-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31511391 Free PMC article.
-
Approaches to hepatitis C treatment and cure using NS5A inhibitors.Infect Drug Resist. 2014 Mar 5;7:41-56. doi: 10.2147/IDR.S36247. eCollection 2014. Infect Drug Resist. 2014. PMID: 24623983 Free PMC article. Review.
-
A role for domain I of the hepatitis C virus NS5A protein in virus assembly.PLoS Pathog. 2018 Jan 19;14(1):e1006834. doi: 10.1371/journal.ppat.1006834. eCollection 2018 Jan. PLoS Pathog. 2018. PMID: 29352312 Free PMC article.
-
Potent Hepatitis C Virus NS5A Inhibitors Containing a Benzidine Core.ACS Med Chem Lett. 2013 Dec 4;5(3):255-8. doi: 10.1021/ml4003293. eCollection 2014 Mar 13. ACS Med Chem Lett. 2013. PMID: 24900814 Free PMC article.
References
-
- Hoofnagle J. H. (2002) Course and outcome of hepatitis C. Hepatology 36, S21–S29 - PubMed
-
- Alter H. J., Seeff L. B. (2000) Recovery, persistence, and sequelae in hepatitis C virus infection. A perspective on long-term outcome. Semin. Liver Dis. 20, 17–35 - PubMed
-
- Di Bisceglie A. M., McHutchison J., Rice C. M. (2002) New therapeutic strategies for hepatitis C. Hepatology 35, 224–231 - PubMed
-
- Tan S. L., Pause A., Shi Y., Sonenberg N. (2002) Hepatitis C therapeutics. Current status and emerging strategies. Nat. Rev. Drug Discov. 1, 867–881 - PubMed
-
- Gao M., Nettles R. E., Belema M., Snyder L. B., Nguyen V. N., Fridell R. A., Serrano-Wu M. H., Langley D. R., Sun J. H., O'Boyle D. R., 2nd, Lemm J. A., Wang C., Knipe J. O., Chien C., Colonno R. J., Grasela D. M., Meanwell N. A., Hamann L. G. (2010) Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 465, 96–100 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

