Novel role of PKR in inflammasome activation and HMGB1 release
- PMID: 22801494
- PMCID: PMC4163918
- DOI: 10.1038/nature11290
Novel role of PKR in inflammasome activation and HMGB1 release
Abstract
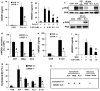
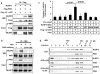
The inflammasome regulates the release of caspase activation-dependent cytokines, including interleukin (IL)-1β, IL-18 and high-mobility group box 1 (HMGB1). By studying HMGB1 release mechanisms, here we identify a role for double-stranded RNA-dependent protein kinase (PKR, also known as EIF2AK2) in inflammasome activation. Exposure of macrophages to inflammasome agonists induced PKR autophosphorylation. PKR inactivation by genetic deletion or pharmacological inhibition severely impaired inflammasome activation in response to double-stranded RNA, ATP, monosodium urate, adjuvant aluminium, rotenone, live Escherichia coli, anthrax lethal toxin, DNA transfection and Salmonella typhimurium infection. PKR deficiency significantly inhibited the secretion of IL-1β, IL-18 and HMGB1 in E. coli-induced peritonitis. PKR physically interacts with several inflammasome components, including NOD-like receptor (NLR) family pyrin domain-containing 3 (NLRP3), NLRP1, NLR family CARD domain-containing protein 4 (NLRC4), absent in melanoma 2 (AIM2), and broadly regulates inflammasome activation. PKR autophosphorylation in a cell-free system with recombinant NLRP3, apoptosis-associated speck-like protein containing a CARD (ASC, also known as PYCARD) and pro-caspase-1 reconstitutes inflammasome activity. These results show a crucial role for PKR in inflammasome activation, and indicate that it should be possible to pharmacologically target this molecule to treat inflammation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. - PubMed
-
- Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. - PubMed
-
- Lamkanfi M, Dixit VM. Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol. 2011;187:597–602. - PubMed
-
- Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

