Oligodendroglia metabolically support axons and contribute to neurodegeneration
- PMID: 22801498
- PMCID: PMC3408792
- DOI: 10.1038/nature11314
Oligodendroglia metabolically support axons and contribute to neurodegeneration
Abstract
Oligodendroglia support axon survival and function through mechanisms independent of myelination, and their dysfunction leads to axon degeneration in several diseases. The cause of this degeneration has not been determined, but lack of energy metabolites such as glucose or lactate has been proposed. Lactate is transported exclusively by monocarboxylate transporters, and changes to these transporters alter lactate production and use. Here we show that the most abundant lactate transporter in the central nervous system, monocarboxylate transporter 1 (MCT1, also known as SLC16A1), is highly enriched within oligodendroglia and that disruption of this transporter produces axon damage and neuron loss in animal and cell culture models. In addition, this same transporter is reduced in patients with, and in mouse models of, amyotrophic lateral sclerosis, suggesting a role for oligodendroglial MCT1 in pathogenesis. The role of oligodendroglia in axon function and neuron survival has been elusive; this study defines a new fundamental mechanism by which oligodendroglia support neurons and axons.
Conflict of interest statement
Figures

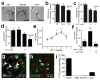
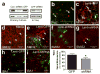
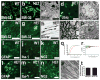
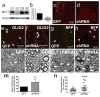
Comment in
-
Neuroscience: The wrap that feeds neurons.Nature. 2012 Jul 25;487(7408):435-6. doi: 10.1038/487435a. Nature. 2012. PMID: 22836992 No abstract available.
-
Glia: Oligodendrocyte transporters feed axons.Nat Rev Neurosci. 2012 Sep;13(9):601. doi: 10.1038/nrn3316. Epub 2012 Aug 1. Nat Rev Neurosci. 2012. PMID: 22850831 No abstract available.
-
Lactate-starved neurons in ALS.Dis Model Mech. 2012 Nov;5(6):711-2. doi: 10.1242/dmm.010892. Dis Model Mech. 2012. PMID: 23115199 Free PMC article.
-
Oligodendroglial dysfunction associated with lactate transport deficiency contributes to neurodegeneration.Mov Disord. 2012 Sep 15;27(11):1357. doi: 10.1002/mds.25177. Mov Disord. 2012. PMID: 23166928 No abstract available.
References
-
- Trapp BD, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. - PubMed
-
- Garbern JY, et al. Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain. 2002;125:551–561. - PubMed
-
- Griffiths I, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. - PubMed
-
- Lappe-Siefke C, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

