Pyk2 cytonuclear localization: mechanisms and regulation by serine dephosphorylation
- PMID: 22802128
- PMCID: PMC11113809
- DOI: 10.1007/s00018-012-1075-5
Pyk2 cytonuclear localization: mechanisms and regulation by serine dephosphorylation
Abstract
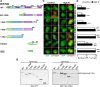
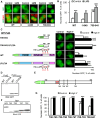
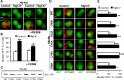
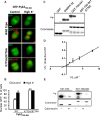
Cytonuclear signaling is essential for long-term alterations of cellular properties. Several pathways involving regulated nuclear accumulation of Ser/Thr kinases have been described but little is known about cytonuclear trafficking of tyrosine kinases. Proline-rich tyrosine kinase 2 (Pyk2) is a cytoplasmic non-receptor tyrosine kinase enriched in neurons and involved in functions ranging from synaptic plasticity to bone resorption, as well as in cancer. We previously showed the Ca(2+)-induced, calcineurin-dependent, nuclear localization of Pyk2. Here, we characterize the molecular mechanisms of Pyk2 cytonuclear localization in transfected PC12 cells. The 700-841 linker region of Pyk2 recapitulates its depolarization-induced nuclear accumulation. This region includes a nuclear export motif regulated by phosphorylation at residue S778, a substrate of cAMP-dependent protein kinase and calcineurin. Nuclear import is controlled by a previously identified sequence in the N-terminal domain and by a novel nuclear targeting signal in the linker region. Regulation of cytonuclear trafficking is independent of Pyk2 activity. The region regulating nuclear localization is absent from the non-neuronal shorter splice isoform of Pyk2. Our results elucidate the mechanisms of Ca(2+)-induced nuclear accumulation of Pyk2. They also suggest that Pyk2 nuclear accumulation is a novel type of signaling response that may contribute to specific long-term adaptations in neurons.
Conflict of interest statement
None.
Figures


Similar articles
-
Calcineurin is essential for depolarization-induced nuclear translocation and tyrosine phosphorylation of PYK2 in neurons.J Cell Sci. 2007 Sep 1;120(Pt 17):3034-44. doi: 10.1242/jcs.009613. Epub 2007 Aug 7. J Cell Sci. 2007. PMID: 17684059
-
Proline-rich tyrosine kinase 2 regulates hippocampal long-term depression.J Neurosci. 2010 Sep 8;30(36):11983-93. doi: 10.1523/JNEUROSCI.1029-10.2010. J Neurosci. 2010. PMID: 20826662 Free PMC article.
-
Involvement of proline-rich tyrosine kinase 2 in platelet activation: tyrosine phosphorylation mostly dependent on alphaIIbbeta3 integrin and protein kinase C, translocation to the cytoskeleton and association with Shc through Grb2.Biochem J. 2000 Apr 15;347(Pt 2):561-9. doi: 10.1042/0264-6021:3470561. Biochem J. 2000. PMID: 10749687 Free PMC article.
-
Differential regulation of FAK+ and PYK2/Cakbeta, two related tyrosine kinases, in rat hippocampal slices: effects of LPA, carbachol, depolarization and hyperosmolarity.Eur J Neurosci. 1998 May;10(5):1667-75. doi: 10.1046/j.1460-9568.1998.00174.x. Eur J Neurosci. 1998. PMID: 9751139
-
Salt-inducible kinase-1 represses cAMP response element-binding protein activity both in the nucleus and in the cytoplasm.Eur J Biochem. 2004 Nov;271(21):4307-19. doi: 10.1111/j.1432-1033.2004.04372.x. Eur J Biochem. 2004. PMID: 15511237
Cited by
-
Pyk2 Regulates MAMs and Mitochondrial Dynamics in Hippocampal Neurons.Cells. 2022 Mar 1;11(5):842. doi: 10.3390/cells11050842. Cells. 2022. PMID: 35269464 Free PMC article.
-
SUPPRESSOR OF APICAL DOMINANCE1 of Sporisorium reilianum Modulates Inflorescence Branching Architecture in Maize and Arabidopsis.Plant Physiol. 2015 Dec;169(4):2789-804. doi: 10.1104/pp.15.01347. Epub 2015 Oct 28. Plant Physiol. 2015. PMID: 26511912 Free PMC article.
-
Functions of the FAK family kinases in T cells: beyond actin cytoskeletal rearrangement.Immunol Res. 2014 Aug;59(1-3):23-34. doi: 10.1007/s12026-014-8527-y. Immunol Res. 2014. PMID: 24816556 Free PMC article. Review.
-
Endogenous Control Mechanisms of FAK and PYK2 and Their Relevance to Cancer Development.Cancers (Basel). 2018 Jun 11;10(6):196. doi: 10.3390/cancers10060196. Cancers (Basel). 2018. PMID: 29891810 Free PMC article. Review.
-
Pyk2 modulates hippocampal excitatory synapses and contributes to cognitive deficits in a Huntington's disease model.Nat Commun. 2017 May 30;8:15592. doi: 10.1038/ncomms15592. Nat Commun. 2017. PMID: 28555636 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

