Abscess formation and alpha-hemolysin induced toxicity in a mouse model of Staphylococcus aureus peritoneal infection
- PMID: 22802349
- PMCID: PMC3457571
- DOI: 10.1128/IAI.00442-12
Abscess formation and alpha-hemolysin induced toxicity in a mouse model of Staphylococcus aureus peritoneal infection
Abstract
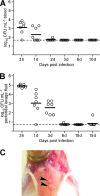
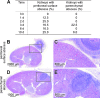
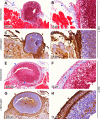
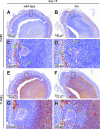
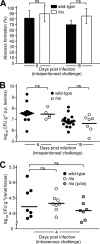
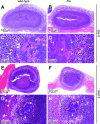
Staphylococcus aureus is a frequent cause of skin infection and sepsis in humans. Preclinical vaccine studies with S. aureus have used a mouse model with intraperitoneal challenge and survival determination as a measure for efficacy. To appreciate the selection of protective antigens in this model, we sought to characterize the pathological attributes of S. aureus infection in the peritoneal cavity. Testing C57BL/6J and BALB/c mice, >10(9) CFU of S. aureus Newman were needed to produce a lethal outcome in 90% of animals infected via intraperitoneal injection. Both necropsy and histopathology revealed the presence of intraperitoneal abscesses in the vicinity of inoculation sites. Abscesses were comprised of fibrin as well as collagen deposits and immune cells with staphylococci replicating at the center of these lesions. Animals that succumbed to challenge harbored staphylococci in abscess lesions and in blood. The establishment of lethal infections, but not the development of intraperitoneal abscesses, was dependent on S. aureus expression of alpha-hemolysin (Hla). Active immunization with nontoxigenic Hla(H35L) or passive immunization with neutralizing monoclonal antibodies protected mice against early lethal events associated with intraperitoneal S. aureus infection but did not affect the establishment of abscess lesions. These results characterize a mouse model for the study of intraperitoneal abscess formation by S. aureus, a disease that occurs frequently in humans undergoing continuous ambulatory peritoneal dialysis for end-stage renal disease.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

