Hematopoietic stem cell development requires transient Wnt/β-catenin activity
- PMID: 22802352
- PMCID: PMC3409499
- DOI: 10.1084/jem.20120225
Hematopoietic stem cell development requires transient Wnt/β-catenin activity
Abstract
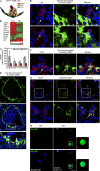
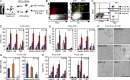
Understanding how hematopoietic stem cells (HSCs) are generated and the signals that control this process is a crucial issue for regenerative medicine applications that require in vitro production of HSC. HSCs emerge during embryonic life from an endothelial-like cell population that resides in the aorta-gonad-mesonephros (AGM) region. We show here that β-catenin is nuclear and active in few endothelial nonhematopoietic cells closely associated with the emerging hematopoietic clusters of the embryonic aorta during mouse development. Importantly, Wnt/β-catenin activity is transiently required in the AGM to generate long-term HSCs and to produce hematopoietic cells in vitro from AGM endothelial precursors. Genetic deletion of β-catenin from the embryonic endothelium stage (using VE-cadherin-Cre recombinase), but not from embryonic hematopoietic cells (using Vav1-Cre), precludes progression of mutant cells toward the hematopoietic lineage; however, these mutant cells still contribute to the adult endothelium. Together, those findings indicate that Wnt/β-catenin activity is needed for the emergence but not the maintenance of HSCs in mouse embryos.
Figures




References
-
- Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D.H., McMahon A.P., Sommer L., Boussadia O., Kemler R. 2001. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 128:1253–1264 - PubMed
-
- Cattelino A., Liebner S., Gallini R., Zanetti A., Balconi G., Corsi A., Bianco P., Wolburg H., Moore R., Oreda B., et al. 2003. The conditional inactivation of the β-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J. Cell Biol. 162:1111–1122 10.1083/jcb.200212157 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

