Origin and control of the dominant time constant of salamander cone photoreceptors
- PMID: 22802362
- PMCID: PMC3409105
- DOI: 10.1085/jgp.201110762
Origin and control of the dominant time constant of salamander cone photoreceptors
Abstract
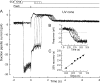
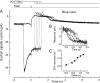
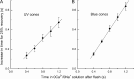
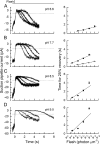
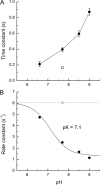
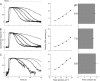
Recovery of the light response in vertebrate photoreceptors requires the shutoff of both active intermediates in the phototransduction cascade: the visual pigment and the transducin-phosphodiesterase complex. Whichever intermediate quenches more slowly will dominate photoresponse recovery. In suction pipette recordings from isolated salamander ultraviolet- and blue-sensitive cones, response recovery was delayed, and the dominant time constant slowed when internal [Ca(2+)] was prevented from changing after a bright flash by exposure to 0Ca(2+)/0Na(+) solution. Taken together with a similar prior observation in salamander red-sensitive cones, these observations indicate that the dominance of response recovery by a Ca(2+)-sensitive process is a general feature of amphibian cone phototransduction. Moreover, changes in the external pH also influenced the dominant time constant of red-sensitive cones even when changes in internal [Ca(2+)] were prevented. Because the cone photopigment is, uniquely, exposed to the external solution, this may represent a direct effect of protons on the equilibrium between its inactive Meta I and active Meta II forms, consistent with the notion that the process dominating recovery of the bright flash response represents quenching of the active Meta II form of the cone photopigment.
Figures



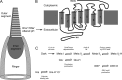
Similar articles
-
Photopigment quenching is Ca2+ dependent and controls response duration in salamander L-cone photoreceptors.J Gen Physiol. 2010 Apr;135(4):355-66. doi: 10.1085/jgp.200910394. Epub 2010 Mar 15. J Gen Physiol. 2010. PMID: 20231373 Free PMC article.
-
The action of 11-cis-retinol on cone opsins and intact cone photoreceptors.J Biol Chem. 2009 Jun 12;284(24):16492-16500. doi: 10.1074/jbc.M109.004697. Epub 2009 Apr 22. J Biol Chem. 2009. PMID: 19386593 Free PMC article.
-
The 9-methyl group of retinal is essential for rapid Meta II decay and phototransduction quenching in red cones.J Gen Physiol. 2009 Aug;134(2):137-50. doi: 10.1085/jgp.200910232. J Gen Physiol. 2009. PMID: 19635855 Free PMC article.
-
Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review.
-
[Physiology of the visual retinal signal: From phototransduction to the visual cycle].J Fr Ophtalmol. 2017 Mar;40(3):239-250. doi: 10.1016/j.jfo.2016.12.006. Epub 2017 Mar 17. J Fr Ophtalmol. 2017. PMID: 28318721 Review. French.
Cited by
-
Circadian regulation of vertebrate cone photoreceptor function.Elife. 2021 Sep 22;10:e68903. doi: 10.7554/eLife.68903. Elife. 2021. PMID: 34550876 Free PMC article.
-
Regulation of mammalian cone phototransduction by recoverin and rhodopsin kinase.J Biol Chem. 2015 Apr 3;290(14):9239-50. doi: 10.1074/jbc.M115.639591. Epub 2015 Feb 11. J Biol Chem. 2015. PMID: 25673692 Free PMC article.
-
Activation and quenching of the phototransduction cascade in retinal cones as inferred from electrophysiology and mathematical modeling.Mol Vis. 2015 Mar 7;21:244-63. eCollection 2015. Mol Vis. 2015. PMID: 25866462 Free PMC article.
-
The Binding Properties and Physiological Functions of Recoverin.Front Mol Neurosci. 2018 Dec 20;11:473. doi: 10.3389/fnmol.2018.00473. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30618620 Free PMC article. Review.
-
Biochemistry and physiology of zebrafish photoreceptors.Pflugers Arch. 2021 Sep;473(9):1569-1585. doi: 10.1007/s00424-021-02528-z. Epub 2021 Feb 17. Pflugers Arch. 2021. PMID: 33598728 Free PMC article. Review.
References
-
- Arnis S., Fahmy K., Hofmann K.P., Sakmar T.P. 1994. A conserved carboxylic acid group mediates light-dependent proton uptake and signaling by rhodopsin. J. Biol. Chem. 269:23879–23881 - PubMed
-
- Bownds D., Dawes J., Miller J., Stahlman M. 1972. Phosphorylation of frog photoreceptor membranes induced by light. Nat. New Biol. 237:125–127 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

