The emerging role of Klotho in clinical nephrology
- PMID: 22802580
- PMCID: PMC3398064
- DOI: 10.1093/ndt/gfs160
The emerging role of Klotho in clinical nephrology
Abstract
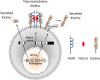
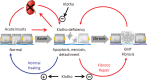
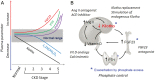
Klotho is highly expressed in the kidney and a soluble form of Klotho functions as an endocrine substance that exerts multiple actions including the modulation of renal solute transport and the protection of the kidney from a variety of insults in experimental models. At present, the Klotho database is still largely preclinical, but the anticipated forthcoming impact on clinical nephrology can be immense. This manuscript puts these potentials into perspective for the clinician. There is renal and systemic Klotho deficiency in both acute kidney injury (AKI) and chronic kidney disease (CKD). Klotho plummets very early and severely in AKI and represents a pathogenic factor that exacerbates acute kidney damage. In CKD, Klotho deficiency exerts a significant impact on progression of renal disease and extra renal complications. In AKI, soluble Klotho levels in plasma and/or urine may serve as an early biomarker for kidney parenchymal injury. Restoration by exogenous supplementation or stimulation of endogenous Klotho may prevent and/or ameliorate kidney injury and mitigate CKD development. In CKD, Klotho levels may be an indicator of early disease and predict the rate of progression, and presence and severity of soft tissue calcification. The correction of Klotho deficiency may delay progression and forestall development of extra renal complications in CKD. Rarely does one find a molecule with such broad potential applications in nephrology. Klotho can possibly emerge on the horizon as a candidate for an unprecedented sole biomarker and intervention. Nephrologists should monitor the progress of the preclinical studies and the imminently emerging human database.
Figures
References
-
- Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. - PubMed
-
- Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. - PubMed
-
- Mitobe M, Yoshida T, Sugiura H, et al. Oxidative stress decreases Klotho expression in a mouse kidney cell line. Nephron Exp Nephrol. 2005;101:e67–74. - PubMed
-
- Ikushima M, Rakugi H, Ishikawa K, et al. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339:827–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

